
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Solve ?
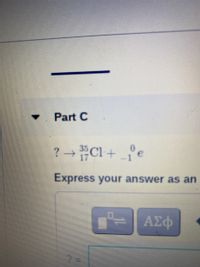
Transcribed Image Text:Part C
? Cl+ °e
Express your answer as an
ΑΣφ
Expert Solution
arrow_forward
Step 1 Analysis

Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A student increases the temperature of a 300 cm³ balloon from 300 K to 600 K. What will the new volume of the balloon be? А. B. 200 cm³ 300 cm³ C. 400 cm³ D. 600 cm³arrow_forwardA student conducts an experiment using an unknown hydrate and obtains the following data Crucible 24.31g Crucible + hydrate 29.31g Crucible + anhydrate 28.26g Further experimentation demonstrates the anhydrate is composed of 29.44% magnesium, 23.55% sulfur and 47.01% oxygen by mass. A. Determine the mass of hydrate, anhydrate and water found in the sample. B. Determine the emperical formula for the anhydrate. C. Determine the formula for the hydrate.arrow_forwardI don't know this question?arrow_forward
- Which of the following is a correct way of measuring the Michaelis constant KM? a. Seconds b. Molecules/second c. Microliters d. Micromolararrow_forwardWhich of the following statements is a feature of scaled particle theory? a) The work required to insert a solute into a solvent depends on the solute's volume only b) The work required to insert a solute into a solvent depends on the solute's accessible surface area only c) High molecular-weight compounds cross biological membranes more easily than low- molecular weight ones d) A large particle has the same surface area-to-volume ratio as a small particle e) Particles in solution pay an energetic penalty to create a cavity in the solventarrow_forwardB. Density of Liquid 1. Obtain a sample of distilled water (any quantity of your choice) anddetermine its mass and volume. Record your data on table 3 in theexperimental report 2. Calculate the density of the water sample using equation 1. Substance Density (g/mL) Substance Density (g/mL) Aluminum 2.7 Lead 11.3 Brass 8.4 Zinc 7.1 Copper 8.9 Cork 0.26 Tin 7.3 Silver 10.5 Bone 1.80 Rhodium 12.4 Iron 7.9 Platinum 21.45arrow_forward
- Show ALL your steps and details in your calculations, by using GRASPS: Given, Required, Analysis, Solution, and Paraphrase. Answer in complete sentences and therefore statements. Show All of your work, in full solutionsarrow_forwardIf given a value that is plot along x-axis: 1. Find given value along x axis. 2. From this point, trace a straight line vertically (parallel to the y-axis) until it intersects with the line graph. 3. Then, trace a line horizontally (parallel to the x axis) from the intersect to the y-axis. 4. The value of y corresponding to the given x value is where the traced line intercepts with the y- axis. Based upon what you see in the graph listed below, estimate the cost of the fence installation. 450 350 A 300 4 250 200 150 100 50 10 151 20 25 30 35 Cost (in $)arrow_forwardWhat is wrong with the following statement? "The results of the experriment do not agree with the theory. something must be wrong with the experiement"arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY