
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
Kindly completes parts A-D:
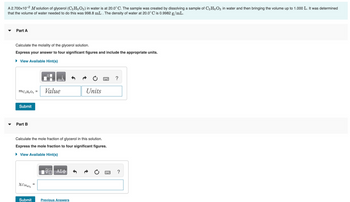
Transcribed Image Text:A 2.700x10-² M solution of glycerol (C3H8O3) in water is at 20.0°C. The sample was created by dissolving a sample of C3H8O3 in water and then bringing the volume up to 1.000 L. It was determined
that the volume of water needed to do this was 998.8 mL . The density of water at 20.0°C is 0.9982 g/mL.
Part A
Calculate the molality of the glycerol solution.
Express your answer to four significant figures and include the appropriate units.
► View Available Hint(s)
MC3H8O3 =
Submit
Part B
XC3H803
Value
Calculate the mole fraction of glycerol in this solution.
Express the mole fraction to four significant figures.
► View Available Hint(s)
=
VE ΑΣΦ
Units
Submit Previous Answers
?
?
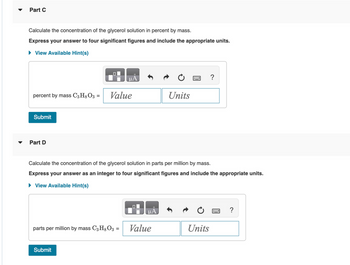
Transcribed Image Text:Part C
Calculate the concentration of the glycerol solution in percent by mass.
Express your answer to four significant figures and include the appropriate units.
► View Available Hint(s)
percent by mass C3H8 O3 =
Submit
Part D
O
Submit
μA
Value
parts per million by mass C3 H8O3 =
Calculate the concentration of the glycerol solution in parts per million by mass.
Express your answer as an integer to four significant figures and include the appropriate units.
► View Available Hint(s)
O
μA
Units
Value
Units
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps with 6 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 5arrow_forwardAcceptable Mechanison? - Œ 50% a I Acceptable Mechanismy- Ph-= -Pr 10215, CH3CH2NH₂ ZonMy Go (31)arrow_forwardte Bb Welcome, Kawtha... Maps News Home GE [Review Topics] [References] 1. Br H,SO, /H,O H20 Mg2+ Br HSO, 2. OH Na HCO, O Na CO2 H20 h = Sy1 Nucleophilic substitution i= SN2 Nucleophilic substitution- j= Electrophilic aromatic substitution a = Proton transfer e = Electrophilic addition b = Lewis acid/base f= El Elimination c = Radical chain substitution d = Radical chain addition g = E2 Elimination Identify the mechanism by which each of the reactions above proceeds from among the mechanisms listed. Use the letters a - j for your answers. 2. Submit Answer Retry Entire Group 9 more group attempts remaining Previous Next Email Instructor Save and Exi Cengage Learning | Cengage Technical Supportarrow_forward
- nouncements CHM240-201 x Organic Chemistry C app.101edu.co 4 72"F Aktiv Chemistry HH OL Mail- Francesca A Tantillo-Out x NCHM 240 F22 Question 15 of 55 Using Cahn-Ingold-Prelog rules, rank these substituents from highest priority to lowest priority. B N A) ||| > | > || B) || > | > III C) II|| > || > | M D) || > ||| > | E) I>II>II N- x + P Update :) Submit 8:56 PM 9/11/2022arrow_forwardChemistry C с dy app.101edu.co 44 # 3 E X | C UL Week 7: Panopto pll III CI $ R F Rank these alkyl halides in order of increasing reactivity in an SN2 reaction. % 5 UL LTU 7-1A T Q CI ^ 6 J Y H X Question 5 of 24 & 7 C|Chegg.com N U A) ||| < | < || B) || < | < ||| C) ||| < || < | D) || < ||| < | E) | < ||| < || PrtScn 8 1 M X + K Home O End A O 2 ENG O 12 ☆ L F10 Tp P PgUp - ¡ * п ( 0 Submin + 6:10 P 10/18/202 ( PgOnarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY