
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
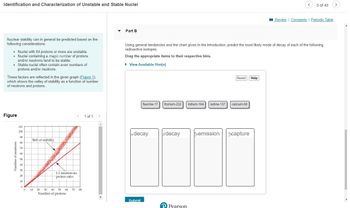
Transcribed Image Text:Identification and Characterization of Unstable and Stable Nuclei
Nuclear stability can in general be predicted based on the
following considerations:
• Nuclei with 84 protons or more are unstable.
Nuclei containing a magic number of protons
and/or neutrons tend to be stable.
• Stable nuclei often contain even numbers of
protons and/or neutrons.
These factors are reflected in the given graph (Figure 1).
which shows the valley of stability as a function of number
of neutrons and protons.
Figure
Number of neutrons
110
100
90
80
70
60
50
40
30
20
10
0
0
Belt of stability
mwing
10 20
ww
1:1 neutron-to-
proton ratio
30 40 50 60 70
Number of protons
80
1 of 1
▼
Part B
Using general tendencies and the chart given in the introduction, predict the most likely mode of decay of each of the following
radioactive isotopes.
Drag the appropriate items to their respective bins.
► View Available Hint(s)
fluorine-17 thorium-232 iridium-164 iodine-137
a decay
Submit
8 decay
P Pearson
Reset Help
calcium-50
< 5 of 43
Peemission capture
Review | Constants | Periodic Table
>
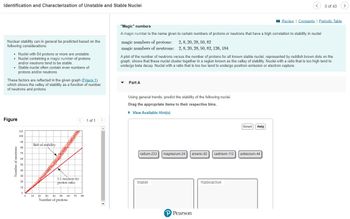
Transcribed Image Text:Identification and Characterization of Unstable and Stable Nuclei
Nuclear stability can in general be predicted based on the
following considerations:
• Nuclei with 84 protons or more are unstable.
• Nuclei containing a magic number of protons
and/or neutrons tend to be stable.
•
Stable nuclei often contain even numbers of
protons and/or neutrons.
These factors are reflected in the given graph (Figure 1).
which shows the valley of stability as a function of number
of neutrons and protons.
Figure
Number of neutrons
110
100
90
80
70
60
50
40
30
20
10
0
0
www
Belt of stability
10
1:1 neutron-to-
proton ratio
20 30 40 50 60 70 80
Number of protons
1 of 1
"Magic" numbers
A magic number is the name given to certain numbers of protons or neutrons that have a high correlation to stability in nuclei:
magic numbers of protons: 2, 8, 20, 28, 50, 82
magic numbers of neutrons: 2, 8, 20, 28, 50, 82, 126, 184
▼
A plot of the number of neutrons versus the number of protons for all known stable nuclei, represented by reddish brown dots on the
graph, shows that these nuclei cluster together in a region known as the valley of stability. Nuclei with a ratio that is too high tend to
undergo beta decay. Nuclei with a ratio that is too low tend to undergo positron emission or electron capture.
Part A
Using general trends, predict the stability of the following nuclei.
Drag the appropriate items to their respective bins.
► View Available Hint(s)
radium-233 magnesium-24 arsenic-82 cadmium-112 potassium-44
Stable
P Pearson
Reset Help
Radioactive
5 of 43
Review | Constants | Periodic Table
Expert Solution
arrow_forward
Step 1
Note - Since it is multiple questions, jece6
Here we have to predict the types of radioactivity radiation from n/p ratio of the following given nuclei.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Calculate the mass defect for the neon-21 nucleus. The mass of neon-21 is 20.993847 u. Please help, I'm confused because my calculations don't check outarrow_forward3 eq req req 2req 2req 3 E D Q C Write a balanced nuclear equation for the decay of bismuth-214 to polonium-214. Submit Answer $ 4 R FL V 07 20 % 5 T [Review Topics] [References] Use the References to access important values if needed for this question. Retry Entire Group 9 more group attempts remaining G Cengage Learning | Cengage Technical Support B 6 5) + T MacBook Pro Y H & 7 N U J * 00 8 + I M ( 9 K ; + "1 { [ 21 Save and Exit ? 11arrow_forwardHow do I answer the equations in a balanced nuclear equation for the beta decay of each of the followingarrow_forward
- q eq # 3 E Q с Based on nuclear stability, what is the symbol for the most likely product nuclide when phosphorus-32 undergoes decay? Submit Answer जी 4 R F V 27 28 % 5 Retry Entire Group 9 more group attempts remaining Use the References to access important values if needed for this question. T G Cengage Learning | Cengage Technical Support A 6 B MacBook Pro Y H & 7 N U J * 00 8 MY M - H ( 9 K Save and Exit ? 11 1 } 1 delearrow_forward▾ Part A Predict a likely mode of decay for each of the following unstable nuclides. Drag the appropriate nuclides to their respective bins. ▸ View Available Hint(s) Beta decay Nb-102 Zr-80 Ru-90 P-27 Submit Request Answer Positron emission Reset Helparrow_forwardLead-210 is a radioactive isotope that sometimes decays through this pathway: ß, α, ß, ß, ß, α. What nuclides form in this decay series? nuclide after the first decay: 210- 83 nuclide after the second decay: nuclide after the third decay: nuclide after the fourth decay: nuclide after the fifth decay: nuclide after the sixth decay: Incorrect Incorrect Incorrect Incorrect Incorrectarrow_forward
- text f5 A % 5 T Calibri 1 16 ¹ 4 1.8 6 - Y 13. The following equation models the beta decay of krypton-89 into rubidium-89. 89 Kr 36 f7 (1) 18 2 & Explain why this reaction is not an example of alpha decay. 7 89 14. Gamma radiation is used to treat certain types of tumors deep in the brain that cannot be accessed with surgery. Suggest a possible reason to explain why gamma radiation is preferred over beta decay or alpha decay for this treatment. fg B U Rb + e - 3 V IUA O fg 8 f10 insert 9 O f11 ☆ O 5 P F12 ▼ $ ✓= prt scr + H 7 G 63°F Cloudy ^ E ] CZ1 delete backspacearrow_forwardWhen the nuclide cesium-129 decays by electron capture: The name of the product nuclide is The symbol for the product nuclide is Submit Answer Retry Entire Group 9 more group attempts remaining s [Review Topics] [References] Use the References to access important values if needed for this question. A Previous ?^arrow_forwardq req 2req بھر میں 3 E D Q C Complete the following nuclear bombardment equation by filling in the nuclear symbol for the missing species. 235U+ on Submit Answer $ 4 R F V % 5 Retry Entire Group 9 more group attempts remaining T [Review Topics] [References] Use the References to access important values if needed for this question. G Cengage Learning | Cengage Technical Support 6 B MacBook Pro Y H & 7 N E U J * 00 8 I M - 0 + Sr+ 2n 9 K e O < B X -O L ** P A - V Previous Email Instructor P { + 11 [ Next Save and Exit 11 ? 1 1arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY