
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
help please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all working
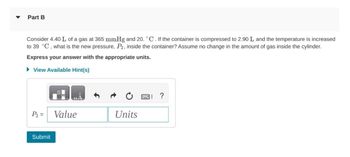
Transcribed Image Text:Part B
Consider 4.40 L of a gas at 365 mmHg and 20. °C. If the container is compressed to 2.90 L and the temperature is increased
to 39 °C, what is the new pressure, P2, inside the container? Assume no change in the amount of gas inside the cylinder.
Express your answer with the appropriate units.
▸ View Available Hint(s)
?
P2 =
Value
Units
Submit
SAVE
AI-Generated Solution
info
AI-generated content may present inaccurate or offensive content that does not represent bartleby’s views.
Unlock instant AI solutions
Tap the button
to generate a solution
to generate a solution
Click the button to generate
a solution
a solution
Knowledge Booster
Similar questions
- Please don't provide handwritten solution .....arrow_forwardHello, I would like help with problem 37 parts A and B in the image attached. Thanks!arrow_forward= teaching and lea x /ilm/takeAssignment/takeCovalentActivity.do?locator-assignment-take juju_ arch CuO chemical name - Google Se xb Answered: A 30.7 ml sample of = x + Free Online Survey... Submit Answer Home - Academia... Create Your Rubric... The Utility Experts .... [Review Topics] [References] Use the References to access important values if needed for this question. A 24.3 mL sample of a 0.318 M aqueous nitrous acid solution is titrated with a 0.405 M aqueous barium hydroxide solution. What is the pH at the start of the titration, before any barium hydroxide has been added? pH = Retry Entire Group 8 more group attempts remaining hp 16 Quando a rede soci... # 0 » NEW * 4x Carrow_forward
- 7. You can see an MSDS below. Please answer the following questions related to the MSDS. a) What is the name of this chemical? b) What should you do if someone drinks the chemical? c) Would this chemical catch on fire if it was exposed to flames? d) If this chemical gets in your eye what should you do? e) What color is this chemical? f) What should you do if someone spills a small amount of the chemical?arrow_forwardGive a clear handwritten answer with explanation..please give answer all sub partsarrow_forwardhelp please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all workingarrow_forward
- chemistry lab please write the answer in the question paper the one I uploaded it thank youarrow_forwardDraw the products of the reactions. products Select Draw Rings More Erase H + H,0 H+ products Select DrawRings More Erase H about us careers privacy policy terms of use contact us helparrow_forwardhelp please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all workingarrow_forward
- session.masteringchemistry.com/myct/itemView?assignment ProblemID=190556628&offset=next Discussion Question 1: Chapter 6 Homework blem 6.20- Enhanced - with Feedback Enter the symbols for the ions and the correct formula for the onic compound formed by each of the following. esc Q A N F1 2 W S F2 X #3 20 E D F3 с S4 $ OOO 000 R Part E F4 F Enter the symbols for the ions of sodium and phosphorus. Enter the lons formed by these elements and separate your answers with a comma (e.g., Sr²+, As³-). cation, anion = Part F Complete previous part(s) Submit Part G cation, anion Submit Part H Complete previous part(s) 65° V Enter the symbols for the ions of calcium and sulfur. Enter the lons formed by these elements and separate your answers with a comma % Request Answer T Request Answer G 6 ΑΣΦ B ΑΣΦ MacBook Air F6 Y H 18⁰ h & 7 N F7 U * CO 8 J BUT RAS poddany ▶II ? F8 1 ? E 9 K F9 O L (e.g., Sr²+, As³-). 0 Ga3+ and 02-E L F10 P F11arrow_forwardhelp please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all workingarrow_forwardEnter the ions present in a solution of K₂CO3. Express your answers as chemical formulas separated by a comma. Offset subscripts and charges on each ion; for charges, write the number before the + or - sign. View Available Hint(s) 5 Provide Feedback Submit esc ΑΣΦ Mother to Son &....pdf A lock ! 1 F1 Q GOOD A @ 2 N 30 F2 W S ? #3 80 F3 X E I дв control option command D A SA 4 Q F4 C R FL O % 5 & F5 T V MacBook Air 6 G F6 Y B & 7 H F7 U N * 8 J DII FB 1 ( 9 M K FO O ) V H 0 < L F10 P commandarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY