
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
need explanation y other options are in correct too
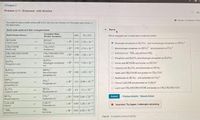
Transcribed Image Text:<Chapter 2
Problem 2.11 - Enhanced - with Solution
56 c
Review I Consarts Peria
You need to make a buffer whose pH is 9.0, and you can choose from the weak acids shown in
the table below
Some weak acids and their conjugale bases
Part A
Conjugate Base
(Proton Acceptor)
K, (M)
Acid (Proton Donor)
pK.
Which conjugate pair is suited best to make this butter?
HCOOH
Formie acid
HCOO
Formate ion
+H
3.75 1.78 x 10
%23
Dihydrogen phosphate on HPO, nd monatydrogen phosphate un IIPO,
CH,COOH
Acatic acid
CH, CO0
Acetate ion
+H476 1.74 x 10
O Monohydrogen phoephale on HPo, and phosehate ion PO,
O Ammonium ion NH, and ammonia NH3
CH,CH(OH)COOH
Lactic acid
CH,CH(OH)COO
Lactate ion
+H3 86 1.38 x 10
H,PO
Phosphoric acid
O Phonphorc acid H, PO, and dhydiogen phosphate ion H; PO
O Formic acid HCOOH and tomate ion HC00
Dinydrogen phosphate +H214 7.24 x 10
ion
O Carbonic acid H,CO, and bicarbonato lon HCO,
H;PO,
Dihydrogen phosphate
ion
HPO,-
Monotrydrogen
phosphate ion
+H6.86 1.38 x 10
O Acetic acid CIH,COOH and acetle lon CH,COO
O Bicartonate ion HCO, and carbonate lon CO,
HPO,
Monohydrogen
phosphate ion
PO,
Phosphate lon
+H 124 3.98 x 10
O Phenoi CH,OH and phenaiate ion C,H,0
O Lactic acid CH,CH(OH)COOH and lactate ion CH, CH(OH)CO0
H; CO,
Carbonic acid
HCO,
+H 63
5.1x 10
Bicarbonate ion
HCO,
co,
Carbonate lon
+H 10.25 5.62 x 10
Piaun Answers Beauest Anawat
Submit
Bicarbonate ion
CH:OH
Phenol
CHSO
Phenolate ion
+H
S89 1.29 x 10-
X Incorrect; Try Again; 4 attempts remaining
NH,
*NH,
Ammonium ion
+H925 5.62 x 10
Ammonia
Part B Complete previous part(a)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY