
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
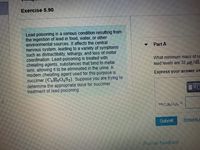
Transcribed Image Text:Exercise 5.90
Lead poisoning is a serious condition resulting from
the ingestion of lead in food, water, or other
environmental sources. It affects the central
nervous system, leading to a variety of symptoms
such as distractibility, lethargy, and loss of motor
coordination. Lead poisoning is treated with
chelating agents, substances that bind to metal
ions, allowing it to be eliminated in the urine. A
modern chelating agent used for this purpose is
succimer (C,H,0,S2). Suppose you are trying to
determine the appropriate dose for succimer
treatment of lead poisoning.
Part A
What minimum mass of su
lead levels are 32 ug/dL
Express your answer us
mC,H,0,S,
Submit
Request
Previde Feedbeck
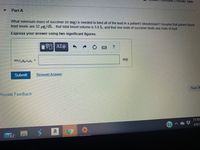
Transcribed Image Text:| Constants T Periodic Table
Part A
What minimum mass of succimer (in mg) is needed to bind all of the lead in a patient's bloodstream? Assume that patient blood
lead levels are 32 ug/dL , that total blood volume is 5.0 L, and that one mole of succimer binds one mole of lead.
Express your answer using two significant figures.
ΑΣφ
mg
= S'O'H'Du
Submit
Request Answer
Next >
Provide Feedback
11:10
4/9/2
a
62
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- ps://openvellum.ecollege.com/course.html?courseld=16594544&OpenVellumHMAC=e0a02f77f7d1 回☆ 21 v Part G Calculate (OH\for a solution formed by adding 4.10 mL of 0.100 M KOH to 10.0 mL of 6.3 x 102M C Ca(OH)2. Express your answer using two significant figures. [OH]]= 0.33 %3D Marrow_forwardYou are asked to prepare a 1.000 L solution of 4.5 M you commit a user error while preparing this solution. Assumed volume Volumetric error Preparation details Added water 2.0 cm above the line, which corresponds to 8.2 mL (0.0082 L) additional solution volume C6H12O6 (glucose; molar mass = 180.16 g/mol) in a lab by dissolving 811.0 g of glucose in water. Consider the following two scenarios in whic Prepared in a beaker Prepared in a volumetric flask 1.000 L You add the glucose to a volumetric flask and then add water until it dissolves. The water bottle you are using has a worn tip, and you inadvertently add too much water such that the meniscus is above the line. The diameter of the neck of the volumetric flask is 2.29 cm. 811.0 g glucose Concentration: You decide to evaluate and compare the errors you made while preparing the solutions using the different methods. Calculate the actual concentrations of the intended 4.5 M glucose solutions prepared by each method based on their…arrow_forwardAn aqueous solution at 25 °C has a H,0' concentration of 7. x 10 °M. Calculate the OH concentration. Be sure your answer has the correct number of significant digits. Show all 9 Discussion week.docX pen dayum.pdf POF 3:51 PM ヘ梦の P Type here to search 5/4/2021 hp 24 to tyo insert prt sc delete 144 esC & 7 % 4. backspace home E R Yarrow_forward
- Chemistry HW need help. How to do these calculation base on the data that is given?arrow_forwardC app.101edu.co Time's Up! What volume in L of a 0.724 M Nal solution contains 0.405 mol of Nal ? X ADD FACTOR ANSWER RESET 2 6.022 x 1023 148.89 559 22.99 g Nal g Nal/mol L Call 1-800-Xfinity. Equip, taxes, fees extra and subi to change ARTING AMOUNT 126.90 olution in milliliters? tv mL MacBook Air 0.724 0.559 1.79 M Nal 0.293 mol Nal A G 1 0.405arrow_forwardshow full and complete procedure HANDWRITTEN onlyarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY