
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:▼
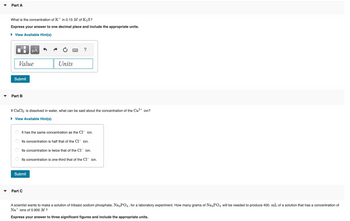
Part A
What is the concentration of K+ in 0.15 M of K₂S?
Express your answer to one decimal place and include the appropriate units.
► View Available Hint(s)
Value
Submit
Part B
μÃ
If CaCl2 is dissolved in water, what can be said about the concentration of the Ca²+ ion?
► View Available Hint(s)
Units
It has the same concentration as the Cl¯ ion.
Its concentration is half that of the C1 ion.
Its concentration is twice that of the C1 ion.
Submit
Its concentration is one-third that of the C1 ion.
Part C
A scientist wants to make a solution of tribasic sodium phosphate, Na3PO4, for a laboratory experiment. How many grams of Na3PO4 will be needed to produce 400. mL of a solution that has a concentration of
Na+ ions of 0.900 M ?
Express your answer to three significant figures and include the appropriate units.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- An aqueous solution of hydroiodic acid is standardized by titration with a 0.179 M solution of barium hydroxide. If 19.1 mL of base are required to neutralize 10.1 mL of the acid, what is the molarity of the hydroiodic acid solution? ____M hydroiodic acidarrow_forwardO Microsoft O shift V A chemistry student weighs out 0.0608 g of formic acid (HCHO₂) into a 250. mL volumetric flask and dilutes to the mark with distilled water. He plans to titrate the acid with 0.1400M NaOH solution. OCHEMICAL REACTIONS Determining the volume of base needed to titrate a given mass... Calculate the volume of NaOH solution the student will need to add to reach the equivalence point. Be sure your answer has the correct number of significant digits. W Microsoft Microsoft 6.52.210... tab esc caps lock mL control Explanation ! 1 (8) Q A Check N © 2 W S #3 X T option command E x10 X D NOV 14 $ 4 C R S F do L % 5 V T G tv ♫ 6 MacBook Pro 9 Y & 7 H Ⓒ2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility U 8 1 9 BN M A O J K O O JOD 05 L H W P > { I command option DOCX + 11 ? | 1 9 OCarrow_forwardfari File Edit View History Bookmarks Window Help DI OD D ¡l A C =5 Previous Page 9 of 9 Next Ⓒ 2 OCT 12 W S S Stv CHAPTER 4-STOICHIOMETRY: QUANTITATIVE INFORMATION ABOUT CHEMICAL REACTION #3 S % by mass E A 10.2 g sample of an aqueous solution of perchloric acid contains an unknown amount of the acid. If 20.5 mL of 1.22 M sodium hydroxide are required to neutralize the perchloric acid, what is the percent by mass of perchloric acid in the mixture? D all A 54 U S R Document3.docx F C ● % 5 C References Use the References to access important values if needed for this question. east.cengagenow.com T с ● FOCUPSia Pxerenc I.Omni Wenarchive G MacBook Pro C Online t... Y 7 H Oct 10. 2022 at 6:04 AM THAN AUD 25 711/7 AL 111.54 AMM U * 3 8 C 9 K 10 KB 747 KR Micros...(.do WAN archivearrow_forward
- NiCl3NiCl3 is a strong electrolyte. Determine the concentration of each of the individual ions in a 0.650 M NiCl30.650 M NiCl3 solution.arrow_forward1. If 10. g of AgNO3 is available, what volume of 0.40 M AgNO3 solution can be prepared? Volume = mL? 2. A 7.52-g sample of a diprotic acid requires 179.0 mL of a 0.750 M NaOH solution for complete neutralization. Determine the molar mass of the acid. g/mol?arrow_forwardWhat is the molar concentration of Na+ ions in 0.0350 M solutions of the following sodium salts in water? NaBr ____ Marrow_forward
- Oo.125. Subject:- Chemistryarrow_forwardWhat is the concentration of F— ions when 266.640 mg of MgF2 (62.3018 g/mol) completely dissociates in 2.442 L of water? Enter the numeric answer only in the units of mM (millimolar). Do not enter the unit.arrow_forwardAcid Base Neutralization of HCI and Ca(OH)2. Use the volume and Molarity information in the table to determine the limiting reagent (acid or base) and how many excess moles of the other reagent had: + CaCl₂ 2 HCI Ca(OH)2 2 H₂O + Mole ratio Volume, m 505 Molarity 5.35 M moles Excess mol Limiting Excess Reagent: Limiting Reagent: 2 505 mL 2.01 M I I I 1 II 个工 mL I I 1 2 Excess Molarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY