
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:ning
X
l?courseld=17811238&OpenVellumHMAC=e3eb583882094a9ca938fe1bad579099#10001
e to search
Chapter 14 Homework
Problem 14.35 - Enhanced - with Feedback
Part A
Course Home
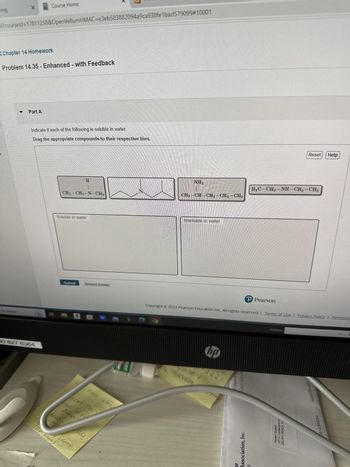
Indicate if each of the following is soluble in water.
Drag the appropriate compounds to their respective bins.
00 827 6354
(EU
BE GOOD,
CH₂-CH₂-N-CH3
Soluble in water
Submit
Request Answer
azel Tor
Scoow
THE INSTE
Shous
bisH- "A GO
*
SHA
NH₂
CH3-CH-CH₂-CH₂-CH3
Insoluble in water
hp
2221
DENTRE D
Copyright © 2023 Pearson Education Inc. All rights reserved. | Terms of Use | Privacy Policy | Permissie
ge
Association, Inc.
P Pearson
HỌC-CH2-NH-CH,-CH,
S
Address
Reset
Hand Dr
Herbert Epstein
14773 Cumberland
DELRAY BEACH,
Help
46-135654
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- help please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all workingarrow_forwardPlease don't provide handwritten solution....arrow_forwardname thisarrow_forward
- 3.) Which arrangement of bonded atoms could explain the high solubility of Compound X in water? IL IV. CH H H CH, H. A. II & III B. III & IV С. I & V D. I onlyarrow_forwardHello, can you help me with this question? Please make sure your answer is not wrong. Thank you.arrow_forwardUsing the appropriate bond energies, calculate the heat of reaction AĦ for the following reaction: Omar bin V CI CI do Data CI-C- CI + H-H- CI-C-CI + H-CI [+] Molal freezing point depression and boiling point elevation constants For example, the Kf and K for water. CI H. [-] Bond energies You can find a table of bond energies by using the Data button on the ALEKS toolbar. energy energy (k)/mol) bond (k)/mol) energy (kJ/mol) Round your answer to the nearest k/mol. bond bond Note: For clarity, all lone pairs have been omitted from the molecular structures. H-H 436 O-H 497 F-F 159 H-F 570 O-F 215 F-a 261 H-CI 431 O-CI 234 F-Br 280 kJ H-Br 366 O-Br 210 F-I 272 mol H-I 298 0-1 213 C-a 243 H-C 413 O-C 385 Cl-Br 219 H-O 497 O=C 532 C-I 211 H-N 391 1077 Br-Br 194 H-P 351 0-0 210 Br-I 179 H-S 381 O=0 498 I-1 152 O-N 206 O=N 631 O=S 551 energy (k/mol) energy (k)/mol) bond energy (kJ/mol) bond bond S-H 381 N-H 391 C-H 413 392 N-C 356 S-F C-F 460 272 S-d N=C 615 C-CI 350 S-Br 230 NEC 749 C-Br 293…arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY