Question
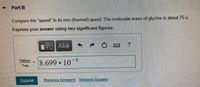
Transcribed Image Text:Part B
Compare this "speed" to its rms (thermal) speed. The molecular mass of glycine is about 75 u.
Express your answer using two significant figures.
να ΑΣφ
Udiffuse
8.699 10 8
Urms
Submit
Previous Answers Request Answer
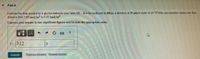
Transcribed Image Text:Part A
Estimate the time needed for a glycine molecule (see Table 13-4 in the textbook) to diffuse a distance of 20 um in water at 20 "Cirts concentration varies over that
distance from 1.05 mol/m' to 0.42 mol/m
Express your answer to two significant figures and include the appropriate units.
HA
312
S
Submit
Previous Answers ReguestAnswer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 5 steps

Knowledge Booster
Similar questions
- I need help solving this problem please help A 6-inch personal pizza has 570 calories. A 14-inch pizza is cut into 8 slices. Estimate the number of calories in one slice of a 14-inch pizza. Round your answer to the nearest calorie.arrow_forwardA surveyor has a stainless steel measuring tape that is calibrated to be 100.000 m long (i.e., accurate to ±1 mm) at 20°C. She needs to add the correction factor to get the true distance. Submit Correct Part B How large, in mm, is the correction factor? Express your answer in millimeters to two significant figures. ► View Available Hint(s) Previous Answers AL = 11 Submit VΠ ΑΣΦ Provide Feedback Previous Answers ? X Incorrect; Try Again; 14 attempts remaining mmarrow_forwardBy how much will the temperature of the tissue increase ? answer in K. show steps on how you solved itarrow_forward
- 1. We have 500 in³ of nitrogen at 1640 psi gauge pressure at 85°F. What is the volume at 1250 psi gauge pressure at 40°F? Show working equation with answer. Answer must be in in^3.arrow_forwardNeeds Complete solution with 100 % accuracy. Don't use chat gpt or ai .arrow_forwardDirections: Solve the following problems. Use g = 9.80 m/s? and assume all numbers are accurate to 3 significant figures unless otherwise indicated. 1. A cylinder that has a 41.0-cm radius and is 50.0 cm deep is filled with air at 29.5°C and 1.00 atm shown in figure (a). A 21.0-kg piston is now lowered into the cylinder, compressing the air trapped inside as it takes equilibrium height hi as shown in figure (b). Finally, a 21.5-kg dog stands on the piston, further compressing the air, which remains at 29.5°C as shown in figure (c). What is the value Ah? Ah 50.0 cm h;arrow_forward
- Can you please answer this question and all of the sub problems show all of the steps to the solutionarrow_forwardPlease Asaparrow_forward1 please need help, keep in mind the 7 basic units and watch the sig figs. Please write in standard form need to see all the numbers in solution thank you so mucharrow_forward
arrow_back_ios
arrow_forward_ios