
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Dd.19.
Transcribed Image Text:▼
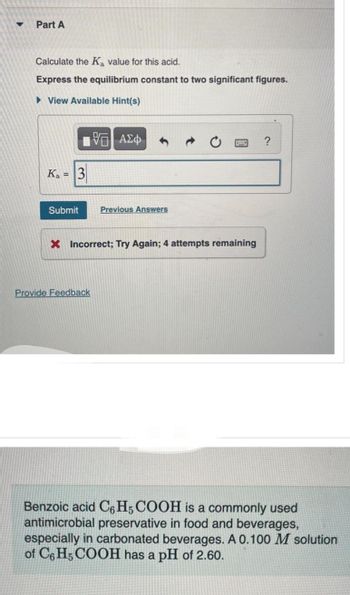
Part A
Calculate the Ka value for this acid.
Express the equilibrium constant to two significant figures.
View Available Hint(s)
Ka 3
—| ΑΣΦ
Submit Previous Answers
X Incorrect; Try Again; 4 attempts remaining
Provide Feedback
?
Benzoic acid C6H5 COOH is a commonly used
antimicrobial preservative in food and beverages,
especially in carbonated beverages. A 0.100 M solution
of C6H5COOH has a pH of 2.60.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 5. a. What is % w/w when 15.5 grams of C6H12O6 dissolves in enough water to make 251 grams of solution? b. Determine mass of the solvent. 6.A What is % w/v when 15.5 ml of C₂H5OH (density = 0.785 g/ml) dissolves in 251 grams of water? B What is % w/w when 15.5 ml of C₂H5OH (density = 0.785 g/ml) dissolves in 251g of solution? CWhat is the molarity of solution, when 15.5 grams of C₂H5OH dissolves in enough water to make up 251 mL of solution? DCalculate ppb and ppm of the above solution 10. Show your calculation for preparation of 230.0 mL of 0.265 M sodium hydroxide solution from 0.850M sodium hydroxide.arrow_forwardAME M Inbox (1,600)-fantil@udeledu X Mail- Francesca A Tantillo-Out x Homepage - CHM150-251 Chen X + → Capp.101edu.co < A Z 89'1 Rain coming 2 S # 3 E D C Uranium hexafluoride, UF, is an important compound used in the enrichment of uranium by gaseous diffusion. $ Which of the following best describes where fluorine can be found on the periodic table? 4 40 R % F 5 B) Noble gases A) Alkaline earth metals C) Lanthanides D) Actinides E) Halogens G O Question 19.b of 23 6 B Aktiv Chemistry OME V Y H * الات & 7 -0✓ INN N PrtScn * 8 J Home End o M ( O 9 K O ) 0 L Alt Pgup m P Pon 12 B ? □ ( 4D Update ***/***/// 512 PM D 7/6/2022 < X Submit Del Backspace Enter Shiftarrow_forwardInbo (534) Conv I Balar b Ansv Post CHE 101 C X The bartl bartl bartl Cher Unkr O Sear E I ma G what app.101edu.co/# Bryant's Gmail Cascadia Canvas Lo... T GSBA Scholarship L... HOMEGROWN TRA... Learn Touch Typing... C The Science of Well... Investor360° ® Login ClickUp Reading list >> Question 31 of 40 Submit A 35.0 mL solution of NaOH is neutralized with 26.5 mL of 0.250 M HCI. What is the concentration of the original NaOH solution? | M 1 4 C 7 8 9 +/- х 100 + 11:15 PM e Type here to search 59°F 8/26/2021 LO (8)arrow_forward
- (4) question is in the photo belowarrow_forward1PrqdNDx-hBYyzKo-xfiM9-Jlq7EAaWe3GBt t3U3jDo/edit ulator O A Land of Permane... it was seconds ago - UA E- EE 2 Al + 3 H,SO, 6. How many grams of hydrogen gas are produced with 0.S8 moles of aluminum sulfate? 3 H, + Al,(SO); 0.88 moles Al (SO), 3 moles H. 6 g H. 0.88 moles Al (SO), 1 mole H.arrow_forwardInbo (534) Conv I Balar b Ansv Post CHE 101 C X с Chec bartl bartl The b My C Unkr O Sear E I ma G what app.101edu.co/# Bryant's Gmail Cascadia Canvas Lo... T GSBA Scholarship L... HOMEGROWN TRA... Learn Touch Typing... C The Science of Well... Investor360° ® Login ClickUp Reading list >> Question 28 of 40 Submit The OH concentration in an aqueous solution at 25 °C is 6.1 x 10 5. What is [H*]? 1 4 C 7 8 9 +/- х 100 + 10:59 PM e Type here to search 59°F 8/26/2021 LO (8)arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY