
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
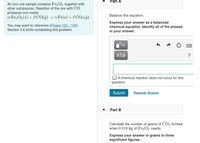
Transcribed Image Text:Part A
An iron ore sample contains Fe2 O3 together with
other substances. Reaction of the ore with CO
produces iron metal:
Balance this equation.
a Fe, O3 (s) + BCO(g) → y Fe(s) + 8 CO2 (g).
You may want to reference (Pages 103 - 105)
Section 3.6 while completing this problem.
Express your answer as a balanced
chemical equation. Identify all of the phases
in your answer.
ΑΣφ
?
O A chemical reaction does not occur for this
question.
Submit
Request Answer
Part B
Calculate the number of grams of CO2 formed
when 0.310 kg of Fe2 O3 reacts.
Express your answer in grams to three
significant figures.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- • BE SURE TO ANSWER AND LABEL ALL PARTS OF EACH QUESTION. • Show all your work. • If you do the work in your head, explain in writing how you did the work. A scientist has three unlabeled samples of pure metals. He wants to determine the identity of each metal. a. Identify which one of the following properties the scientist should use to determine the identity of the pure metal in each sample: color, melting point, mass, or volume. b. Explain why the property you identified in part (a) can be used to determine the identity of the pure metal in each sample. The scientist cuts each of the samples of pure metal into two smaller pieces. c. Is the property that is used to determine the identity of the metal affected when each sample is cut into two pieces? Explain your answer The scientist can also use density to determine the identity of the pure metal in each sample. d. Describe how the scientist can determine the density of the pure metal in each sample.arrow_forwardToxic Cr(VI) can be precipitated from an aqueous solution by bubbling SO₂ through the solution. How much SO₂ is required to treat 3.00 × 108 L of 5.00×10-² mM Cr(VI)? 2Cro +3SO₂ + 4H+ →>> Cr₂(SO4)3 + 2H₂O 1.59 x 10 3 kg →arrow_forwardWrite a balanced chemical equation for the dissociation of lithium bromide in water?(include phases) Write a balanced chemical equation for the dissociation of sodium nitrate in water? (include phases) Write a balanced chemical equation for the dissociation of iron(III) chloride in water? (include phases)arrow_forward
- 91 D. Calculate the moles of NaOH used in each reaction. In Reactions 1 and 2, this can be found from the mass of the NaOH. In Reaction 3, it can be found using the molarity of the sodium hydroxide solution and its volume. Show your calculations and place your data in the Data Sheet. Imid NaOH 100 40 . OS2 Ind N2011 102,1/1000 Oilcl 4or Na011 1051 E. Calculate the moles of HCl used in reactions 2 and 3 using molarity and volume. Show your calculations and place your data in the Data Sheet. L trement errors), identify, one reason L (in other words, don't just 0.052 identif mit reactant ater that into Dato to She marrow_forwardAspirin can be made in the laboratory by reacting acetic anhydride (C4H6O3) with salicylic acid (C7H6O3) to form aspirin (C9H8O4) and acetic acid (C2H4O2). The balanced equation is:C4H6O3+C7H6O3→C9H8O4+C2H4O2.In a laboratory synthesis, a student begins with 5.00 mL of acetic anhydride (density = 1.08 g / mL) and 2.08 g of salicylic acid. Once the reaction is complete, the student collects 2.14 g of aspirin. -Determine the limiting reactant for the reaction.Express your answer as a chemical formula. -Determine the theoretical yield of aspirin for the reaction. -arrow_forward7. Applying the principles of green chemistry requires some consideration of the way in which they can be applied. Figure 1 represents a generic industrial process. Using the principles of green chemistry and circled letters, describę how four of the principles could be applied to this process to make it a green operation. E kaEA product raw materials energy Source waste гeactor Figure 1 starrow_forward
- A solution containing 78 g of NaNO3 in 70. g H2O at 50 ° C is cooled to 20 °C. Use the solubility data from the table below. Part A Solubility (g/100. g H2O) Substance 20 °C 50 °C How many grams of NaNO3 remain in solution at 20 °C? KCI 34 43 Express your answer in grams to two significant figures. NaNO3 88 110 ? C12 H22O11 (sugar) 204 260arrow_forwardSuppose you are brewing beer, and you want to estimate how long it will take to complete the fermentation process. You place1.00 kgof sucrose(MM=342.3 g/mol)and yeast in your fermentation vessel and add water to a final volume of3.50 L. You decide that your reaction will be considered completed when the concentration of sugar decreases to0.0100M. How long will it take (in minutes) from the start of the reaction to the completion of the reaction?arrow_forwardProvide the appropriate product for the examplearrow_forward
- You were tasked to separate the components of a mixture containing silica, sodium chloride and charcoal. TNāCI dissolves in water while silica and charcoal are not water-soluble. Only charcoal dissolves in carbon disulfide. a. Write a short experimental procedure to carry out the separation of the mixture. b. Given the following data, determine the percentage of charcoal, sodium chloride and silica. Mass (g) Mass of beaker 100.000 Mass of beaker + mixture 110.000 mass of evaporating dish 62.000 mass of evaporating dish + solid after evaporation of water 65.000 Mass of beaker + charcoal + silica after evaporation of excess water 117.000 mass of beaker + silica after decanting dissolved charcoal and drying 113.545arrow_forwardSodium nitrate, NaNO3, cannot be analyzed gravimetrically because a. the stability of sodium nitrate is very low. b. sodium nitrate is insoluble. c. all compounds containing sodium ions or nitrate ions are soluble. d. sodium nitrate is an inert substance.arrow_forwarddiagram b is correct, What procedures can be used to solve this problem? write down the plan for this new way to solve the problem. 1. first execute plan 1 to solve this problem. explain in every step. 2. solve the problem again - this time use the plan that you propose, plan 2. 3. finally, think about the way that you can use it to make sure that your answer makes sense. write your assessment.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY