
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Need assistance with part 4 & 5 please
“First, draw the expected product from the reaction. Second, draw the stepwise mechanism that shows the formation of the product. Please use stereochemistry in Part 4”
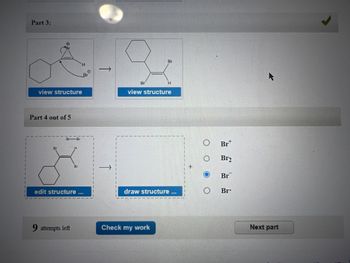
Transcribed Image Text:Part 3:
view structure
Part 4 out of 5
e
Br
Br
view structure
Br
Br₂
+
Br
edit structure
draw structure ...
Br
9 attempts left
Check my work
Next part
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 5 images

Knowledge Booster
Similar questions
- determine the molecular structure based on the spectroscopic data in the picture explain for each picturearrow_forwardA What Hb Exper Molec Mw Cordi y! techn y samp BrFs html?courseld%3D15828855&HE ID=537061946710f71a192e43597b01c6e0#10001 Principles of Chemistry I (Chem 1107-327) Report ab Report Indicate which structures have dipole moments and show their direction. Br F Br-F Br-F Yes Yes Yes L No Dipole Yes Yes 99+arrow_forwardG Mgert x O Phyal-Seren, x Menge PRACTICAL EAND O on tta dutex O Fik | CUsers/NolyDownloady/Physical-Science Q1.Mod2.paf Guest = Physical-Scienoe_01_Modz.pdl 12 / 16 100% Miscilility Activity 2 Molecular Doodle Directions: lustrate the Lewis dot atruetures, name the shape of the molecuir, and identify whether the following molecules are polar ar nonpolar based an 10 strucnure. Molecule/Compound AICI Lewin Dot Strueture Shape Polarity NO PCl CO, HCN CH.arrow_forward
- 89-understanding periodic trendsarrow_forwardmart - Hiring Ce... 3 441) # 3 H—N—H E D C F4 $ 4 The electron-domain geometry and molecular geometry of ammonia are and respectively. The Lewis structure is given below. R F F5 I-Z: V H ► 11 % 5 F6 T G B A 6 F7 Y H I F8 & 7 N 8 Question 3 of 4 J F9 * > 8 DELL 8. F10 D M K ( 9 < E Alt A) tetrahedral, trigonal pyramidal O B) trigonal planar, trigonal planar C) octahedral, trigonal planar D) T-shaped, trigonal planar F11 * L ) O F12 P : Ctri * PrtScr 2⁰ =1 1E3 ? + { 9 [ I Insert 3:23 PM WD40 4/28/2023 } 1 Delete Backspace ↑ PgUp PgDn Num Lock B Enter Subm Shift 7 E I 4arrow_forwardPLS HELP VERY URGENT. Explanation is not requiredarrow_forward
- Polypropylene Draw the molecule on the canvas by choosing buttons from the Tools (for bonds), Atoms, and Advanced Template toolbars. The single bond is active by default. EXP. CONT. i ? H C S CI Br Marvin JS [1] by O ChemAxon F -arrow_forwardIn the first picture, it should have the questions I answered and hopefully helps a bit on the question I have. I can’t find the electrons for the second picture in the blank yellow highlight.arrow_forwardFind the structurearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning
