
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
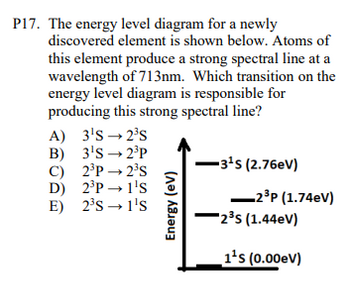
Transcribed Image Text:P17. The energy level diagram for a newly
discovered element is shown below. Atoms of
this element produce a strong spectral line at a
wavelength of 713nm. Which transition on the
energy level diagram is responsible for
producing this strong spectral line?
A) 3¹S-2³S
B) 3¹S-2³P
C) 2³P 2³S
D) 2³P→ 1¹S
2³S→ 1¹S
E)
Energy (eV)
-3¹S (2.76eV)
-2³P (1.74eV)
2³S (1.44eV)
1¹s (0.00eV)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Below are the line spectra of Neon (Z = 10) and Lithium (Z = 3). Which of the following statements best explains why more spectral lines are observed for Neon as compared to Lithium? Neon (Ne) Lithium (Li) Select one: O a. Metals are held by stronger metallic bonds which makes emission more difficult. O b. O c. O d. Neon has more energy levels and electrons which allows more electronic transitions. Neon tends to fluoresce in nature thus more spectral lines are observed. Lithium requires more energy to excite because it prefers to form cations to achieve octet configuration. 19arrow_forwardf,g,h pleasearrow_forward1) Write the full and noble gas configuration for Se. Full Electron Configuration: Response Noble Gas Configuration: 2) The element represented by the following electron configuration is ____________. [Rn] 7s2 5f11 A)Rb B) Es C) Au D)Cf 3)Write the full and noble gas configuration for Cs. Full Electron Configuration: Noble Gas Configuration: please answer the three question correct pleasearrow_forward
- Match the elements to their correct electron configuration: A: [Kr] 4d³ 5s² B: [Ne] 3s² 3p5 C: [Kr] 4d8 5s² D: [Xe] 4f14 5d7 6s² CI Ir Nb Pd [Choose] Choose [Choose] [Choose] Larrow_forwardQ2arrow_forwardPart A By referring only to the periodic table, arrange the atoms in order of increasing atomic size: N, F, P, Ca. ON< Farrow_forward
- JI A student draws the orbital diagram below for the 3p electrons in an S atom. What, if anything, is incorrect about the drawing? NJ 3p A) It violates the Pauli exclusion principle. B) It violates Hund's rule. C) It violates the Aufbau principle. D) It violates the Heisenberg uncertainty principle. E) There is nothing incorrect about the drawing.arrow_forward1. What color of light is emitted when an excited electron in the hydrogen atom falls from: a) n =5 to n = 2 c) n = 3 to n = 2 b) n = 4 to n = 2arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY