
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
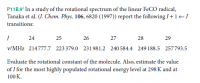
Transcribed Image Text:P11B.9* In a study of the rotational spectrum of the linear FECO radical,
Tanaka et al. (J. Chem. Phys. 106, 6820 (1997)) report the following J+1-J
transitions:
24
25
26
27
28
29
v/MHz 214777.7 223379.0 231981.2 240 584.4 249 188.5 257793.5
Evaluate the rotational constant of the molecule. Also, estimate the value
of J for the most highly populated rotational energy level at 298 K and at
100 K.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- Q1) Calculate the energy of photon required to exite CO molecule from v=0 to v=1, the wavenumber = 2143 cm-1arrow_forwardA Morse oscillator, V(r) = D[1 − e−a(r—ro)]², has D = 164 kJ/mole and w→ ve 1250 cm-1. Compute the energy level (in cm-1) corresponding to the eigenstate shown below. = (Note that the energy units, 11.9627 J/mole = 1 cm-1. It might be helpful to convert the value of D to units of cm-1 before you evaluate the energy level.) wwwarrow_forwardEstimate the vibrational frequency and rotational constant for DBr. Provide a numerical answer in cm-1. Assume the bond length is 1.945 Å and the reduced mass is 1.95 amu.arrow_forward
- For 'H³°CI molecule, (N1/No) ratio at 300 K is 6.091 x 10² . Calculate the wavenumber ( in cm') for J=2→J=3 transition in the microwave spectrum of the molecule.arrow_forwardPlease don't provide handwriting solutionarrow_forward1. Water shows the following vibrations: A₁ symmetrical stretch at 3657 cm ¹, B2 asymmetrical stretch at 3756 cm ¹, A₁ deformation at 1595 cm ¹¹. Determine which vibrations are active in IR absorption spectra and which are active in Raman spectra.arrow_forward
- Infrared absorption by 1H127I gives rise to an R branch from v = 0. What is the wavenumber of the line originating from the rotational state with J = 2? Hint: Use data from Table 11C.1.arrow_forwardThe vibrational transtion from v=0 to v=1 in CO occured at wavenumber= 2143.4 cm-1 calculate the force constant of COarrow_forwardQI.P3 (a) Suppose that the you measure the lowest-energy rotational transition for 160'H at a frequency of 37.742 cm-1. What is the bond length in units of pm? (Show calculation) (b) Suppose that you have a symmetric rotor with rotational constants: A = 125 cm-1 B = 100 cm-1 (HINT: Recall that A is the coefficient along the unique direction.) Assuming that the excitation laser is at 10.000 cm1, calculate all the Anti-Stokes lines involving the J = 0, J = 1, and J = 2 states.arrow_forward
- ▼ ▼ Part A In the rotational spectrum of H³5Cl (I= 2.65 x 10-47 kg m²), the transition corresponding to the J = 4 to J = 5 transition is the most intense. At what temperature was the spectrum obtained? Express the temperature in kelvins to three significant figures. ΠΫΠΙ ΑΣΦ T = Submit Part B J= Request Answer At 1000. K, which rotational transition of H³5 Cl would you expect to demonstrate the greatest intensity? Express your answer as an integer. V—| ΑΣΦ Submit Request Answer K ?arrow_forwardWhat is the difference in population of 13C spins between the upper and lower states in a magnetic field at 2T and 300K?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY