
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
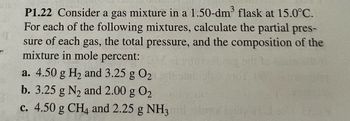
Transcribed Image Text:P1.22 Consider a gas mixture in a 1.50-dm³ flask at 15.0°C.
For each of the following mixtures, calculate the partial pres-
sure of each gas, the total pressure, and the composition of the
mixture in mole percent:
a. 4.50 g H₂ and 3.25 g 0₂
b. 3.25 g N₂ and 2.00 g 0₂
c. 4.50 g CH4 and 2.25 g NH3mil olma
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 6 steps with 44 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Bhumiarrow_forwardHow many moles of hydrochloric acid are needed to produce 26.3 L of chlorine gas according to the following reaction at 0 °C and 1 atm?hydrochloric acid (aq) + oxygen(g)water (l) + chlorine(g) moles hydrochloric acidarrow_forwardPls help me with the following asap and make sure its 100% correct, thanksarrow_forward
- A sample of gas is found to exert a pressure of 7.00 x 104 when it is in a 3.00L flask at 0.00 deg C calculate the following a) the new volume if p becomes 1.01 x 105 Pa and T is unchanged b) the new pressure if V becomes 2.00L and T is unchanged c) the new pressure if the temperature is raised to 50.00 deg C and V is unchangedarrow_forwardWhat temperature (in oC) is necessary for 2.40 moles of an ideal gas in a 4.50L container to have a pressure of 35.0 atm? Group of answer choices 272 526 811 .0228arrow_forwardThe fermentation of glucose (wine making) reaction is shown below. During this process 780 mL of CO2 gas was produced at 37 oC and 1.00 atm. (R= 0.0821 LatmK-1mol-1) C6H12O6(s) + 6O2(g) à 6CO2 (g) + 6H2O (l) a. Calculate the number of moles of CO2 produced in the above reaction? b.What is the final volume, in liters, of the CO2 gas when measured at 22 oC and 675 mm Hg. ( 1 atm = 760 mm Hg)arrow_forward
- Air bags inflate from the decomposition of sodium azide: 2 NaN3 --> 2 Na + 3 N2 What mass of sodium azide must be reacted to inflate a 70.0 L airbag at a temperature of 55C and 1 atm of pressure? A.112.7 gramsNaN3 B.320 gramsNaN3arrow_forward15.0 L of an ideal gas at 298 K and 3.3E atm are heated to 383 K with a new pressure of 605 ntraarrow_forwardesc G ← D 7 Q A 2 N openvellum.ecollege. X C S + 84°F Mostly cloudy 2 One way to measure temperature in some applications is to monitor the gas pressure in a rigid, closed container. What is the final temperature (in °C) of such a vessel, calibrated to initially read 1.00 atm at 0.00 °C, if the pressure changes to 1.18 atm? # 3 X https://app.101edu.co * W E S D Aktiv Chemistry 14101 $ C 4 R LLI F % X Navy Federal Credit Ur X b Success Confirmation X 5 T Q Search G 40 6 Y V B 4- & 7 H * Question 33 of 33 4+ U N 8 - hp 144 ( 9 M ho 411 ) O Orie way to measure X A ☆ O P - KLE C One way to m 1 4 7 +/- ▷ 2 5 8 brt scarrow_forward
- 5.99. What pressure is exerted by a gas mixture containing 2.00 g of H₂ and 7.00 g of N₂ at 273°C in a 10.0 L container? What is the contribution of N₂ to the total pressure? A 70 CAT 5.100 CITTarrow_forwardIn the reaction 2SO2(g) + O2(g)--> 2SO3(g) how many liters of SO3 gas at STP can be produced from 4 liters of SO2 and 3 liters of O2 all at STP?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY