
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
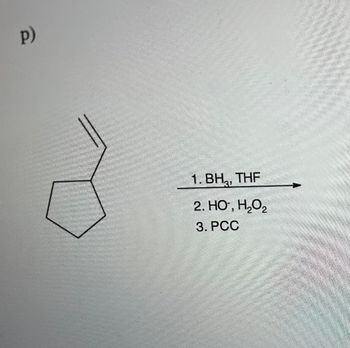
Transcribed Image Text:p)
1. BH, THF
2. HO, H₂O₂
3. PCC
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Write a balanced equation for the reaction of nitric acid, HNO3(aq), with magnesium hydroxide, Mg(OH)2(aq). Include physical states.arrow_forwardBalance the equation. Fill in the blank Mg(OH)2 HCI MgCl2 + H20arrow_forwardDetermine the mass in grams of 3.00 × 10²¹ atoms of arsenic. (The mass of one mole of arsenic is 74.92 g.) Determine the formula weight of Al₂(SO₄)₃. Provide an answer to two decimal places. How many moles of Ag are in 43.6 grams of Ag₂O? 100.0 mL of a 0.500 M solution of KBr is diluted to 500.0 mL. What is the new concentration of the solution?arrow_forward
- I need D-Harrow_forwardPredict the reactants of this chemical reaction. That is, fill in the left side of the chemical equation. Be sure the equation you submit is balanced. (You can edit both sides of the equation to balance it, if you need to.) Note: you are writing the molecular, and not the net ionic equation. → NaCIO, (aq) + H₂O(1) X ローロ Śarrow_forward3. For each acid, name the anion bonded to hydrogen, then name the acid. Anion Name Acid Name (а) НBr (b) НF (c) H;S (d) HNO; (e) HC10;arrow_forward
- Balance: HC2H3O2(aq)+ Al(OH)3(s)arrow_forwardBa(ClO3)2 → ____ + 3O2 In one or two sentences, identify the type of reaction that is occurring and identify the other product. Based on the subscripts of the elements in the other product and the fact that chlorine has seven valence electrons, determine the number of electrons in barium’s (Ba) outer shell.arrow_forwardList the spectator ions of Na2CO3(aq) + Ca(NO3)2(aq) → CaCO3(s) + 2NaNO3(aq)arrow_forward
- A chemist adds 2.00 L of a 5.9 × 10M mercury(II) iodide (HgI₂) solution to a reaction flask. Calculate the moles of mercury(II) iodide the chemist has added to the flask. Round your answer to 2 significant digits. mol 0 X Sarrow_forward8. Calculate the mass, in grams, of 0.433 mol of CO2.arrow_forwardABS plastic is a polymer consisting of three monomer units: acrylonitrile (C₃H₃N), butadiene (C₄H₆), and styrene (C₈H₈). A sample of ABS plastic contains 11.9% N by mass. It took 1.56 g of Br₂ to react completely with a 2.00 g sample of ABS plastic. Bromine reacts 1:1 (by moles) with the butadiene units in the polymer and nothing else, so bromination is a method for determining the quantity of butadiene in the polymer. What is the percent by mass of styrene in the polymer?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY