
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
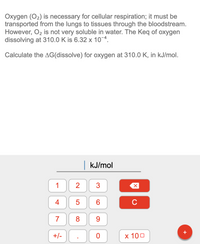
Transcribed Image Text:Oxygen (O2) is necessary for cellular respiration; it must be
transported from the lungs to tissues through the bloodstream.
However, O2 is not very soluble in water. The Keq of oxygen
dissolving at 310.0 K is 6.32 x 10¬4.
Calculate the AG(dissolve) for oxygen at 310.0 K, in kJ/mol.
|kJ/mol
1
3
4
6
C
7
9
+/-
х 100
+
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A 30.0 mL aliquot of 0.300 M H_3 PO_4 is mixed with 90.0 mL of 0.200 M KOH, and the mixture is evaporated. Will the salt that crystallizes out be K_3 PO_4, K_2 HPO_4, or KH_2 PO_4? Show your calculations by using an ICE table (or two or three). H_3 PO_4(aq) + KOH_(aq) rightarrow KH_2 PO_4(aq) + H_2 O (l) The next proton on the H_2 PO_4^- would start to react only when more OH^- was added, over and above the first mole: KH_2 PO_4(aq) + KOH_(aq) rightarrow K_2 HPO_4(aq) + H_2 O (I) The final proton on the HPO_4^2- would start to react only when more OH^- was added, over and above the second mole: K_2 HPO_4(aq) + KOH_(aq) rightarrow K_3 PO_4(aq) + H_2 O (I)arrow_forward1. Use the AG = AH – TAS relationship to justify why the solubility of KOH(s) in water decreases as temperature is increased. Do not do any calculations. (HINT: the S° value for KOH(s) is greater than that for KOH(aq).)arrow_forwardA solution was prepared by dissolving 0.687 g of sulfur, S, in 100.0 g of acetic acid, HC2H3O2. Calculate the freezing point and boiling point of the solution. (K, = 3.08°C/m and Kf = 3.59°C/m, T, = 118.5°C and T† = 16.60°C) Freezing point: °C Boiling point: °Carrow_forward
- Q/ Mohr's method uses the potassium chromate indicator and this indicator makes the equivalent point not identical to the end point. A sample weighing 5.592 g of BaCl2 is dissolved in 500 mL of water. Calculate the percentage of chloride in the sample when 25 mL of the sample is titrated and requires 33.47 mL of 0.1098 N AGNO. SOLUTION:arrow_forwardThe solubility of CO2 in water is a function of pressure.Calculate the concentration of CO2 in water when ???2 = 2.5 × 10−4 bararrow_forward6arrow_forward
- 4 g The solubility of PbCrO, in water at 25 °C is measured to be 1.7 x 10 Use this information to calculate K, for PbCrO4. L sp Round your answer to 2 significant digits.arrow_forwardWhat weight of potassium hydrogen phthalate will require 30. mL of a 0.30M NaOH solution to reach the equivalence point? KHP MM 204.22 g/mol .CO;K' .CO;K' NaOH + H2O + *CO,H COƠ,Na'arrow_forwardConsider the following solutions: (i) 400.0 mL of 0.10 M NaCl (ii) 300.0 mL of 0.10 M CaCl2 (iii) 200.0 mL of 0.10 M FeCl3 (iv) 200.0 mL of 0.10 M KBr (v) 800.0 mL of 0.10 M sucrose Solution (v), the sucrose solution, has the greatest number of ions. Solution (iii), the FeCl3 solution, has the greatest number of ions. Solution (iv), the KBr solution, has the greatest number of ions. Solution (i), the NaCl solution, has the greatest number of ions. Solution (ii), the CaCl2 solution, has the greatest number of ions.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY