
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:8 of 13
I Review I Constants
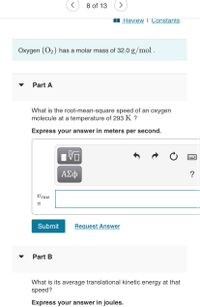
Oxygen (O2) has a molar mass of 32.0 g/mol .
Part A
What is the root-mean-square speed of an oxygen
molecule at a temperature of 293 K ?
Express your answer in meters per second.
ΑΣφ
?
Vrms
Submit
Request Answer
Part B
What is its average translational kinetic energy at that
speed?
Express your answer in joules.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- The study of distributions of carbon dioxide (CO₂) and carbon monoxide (CO) in the atmosphere is an important environmental issue. The masses of CO₂ and CO molecules are 44u and 28u, respectively with u = 1.66x10-²7 kg. A. Calculate the most probable speed, average speed, and rms speed for CO₂ and CO molecules at 290K. B. Discuss the possible distributions of these particles throughout the atmosphere with explanations.arrow_forwardModern vacuum pumps make it easy to attain pressures of the order of 10-13 atm in the laboratory. For related problem-solving tips and strategies, you may want to view a Video Tutor Solution of Atomic and molecular mass. Part A At a pressure of 6.20x10-14 atm and an ordinary temperature of 296.0 K, how many molecules are present in a volume of 1.08 cm³ ? Enter your answer numerically. N = Submit Part B IVE ΑΣΦ N = Request Answer ? How many molecules would be present at the same temperature but at 1.04 atm instead? Enter your answer numerically. VE ΑΣΦ molecules ? moleculesarrow_forwardA container of nitrogen molecules is at a temperature of37.0 C. a. What is the rms speed of the nitrogen molecules?b. What is the average rotational energy of a nitrogen molecule?arrow_forward
- 4.0 moles of krypton gas are in a 0.20m3 container. The pressure is 1.663 x 105 Pa. a, What is the temperature (to the nearest K)? The volume contracts to 0.10m3. The pressure is held constant. b, How much work was done by the gas during the volume contraction? c, What is the temperature after the volume contraction (to the nearest K)?arrow_forwardAssume a person has lungs that can hold V = 5.5 L of air (at 1 atm), and their body temperature is currently T = 35.5° C. a. How many moles of air does the average human have in their lungs when they breath in?arrow_forwardFor a planet to have an atmosphere, gravity must be sufficient to keep the gas from escaping The escape speed a particle needs to escape the earth's gravitational attraction is 1.1 x 10 m/s. The motion of projectiles never depends on mass, so this escape speed applies equally to rockets and to molecules in the earth's upper atmosphere. Part A At what temperature does the rms speed of nitrogen molecules equal the escape speed? Express your answer in kelvins. ΜΕ ΑΣΦΑ T- Submit Part B T- Request An At what temperature does the mms speed of hydrogen molecules equal the escape speed? Express your answer in kelvins. VAZO Submit ? K Karrow_forward
- 7 of 13 At an altitude of 11,000 m (a typical cruising altitude for a jet airliner), the air temperature is -59.0° C and the air density is 0.467 kg/m³. The molar mass of air is 28.8 g/mol. Part A What is the pressure of the atmosphere at that altitude? (Note: The temperature at this altitude is not the same as at the surface of the earth, so the equation Р— Ро ехp (- Mgy doesn't apply.) RT Express your answer with the appropriate units. HA ? Value Units Submit Request Answerarrow_forwardAt what temperature (in Kelvin) will the diffusion coefficient for the diffusion of species A in metal B have a value of 5.92 × 10-16 m2/s, assuming values of 2.8 x 10-6 m?/s and 225,000 J/mol for Do and Qd, respectively? i Karrow_forwardRe 460 J of work must be done to compress a gas to half its initial volume at constant temperature. Part A How much work must be done to compress the gas by a factor of 7.00, starting from its initial volume? Express your answer with the appropriate units. HẢ W Value Units %3Darrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON