
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Is this correct
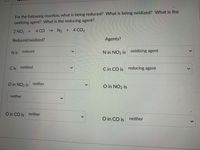
Transcribed Image Text:For the following reaction, what is being reduced? What is being oxidized? What is the
oxidizing agent? What is the reducing agent?
2 NO2 +
4 CO
- N2 + 4 CO2
Reduced/oxidized?
Agents?
N is reduced
N in NO2 is oxidizing agent
C is
oxidized
C in CO is reducing agent
O in NO2 is
neither
O in NO2 is
neither
O in CO is neither
O in CO is
neither
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- tab File esc ns lock Ooop X 39 c) x Gheat heat X Ⓒliquid X N. AskYX N. AskYX A ALEK X b STAT X CI Che X C www-awu.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-IvTqeviKFP6W0cqJcWJdIACROQwyw24GWHIn7gZDVjOY1PIGdwmoRjhTWafIMXzDVQ21WWbzcCZqUs09eN... Omall O YouTube Maps View History bookmarks Profiles 180 window Help O STATES OF MATTER Sketching a described thermodynamic change on a phase... pressure (atm) aleks X 1 Explanation ← v The pressure on a sample of pure X held at -40. °C and 2.09 atm is decreased until the sample melts. The pressure is then held constant and the temperature is increased by 61. °C. On the phase diagram below draw a path that shows this set of changes. Q A 2 Z 200 temperature (K) Check W 400 S # 3 X C с H E D 600 $ 4 C X R F % 5 S G Search or type URL stv V T F MacBook Pro 6 G 4 Y B & 7 H ☆ U N * 8 J + O▬▬▬▬0/5 Ⓒ2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility XO AO W ( 9 M ( @ K O 0 Calcu X < 1 He 60% L M Math X Aa Wed…arrow_forwardThe following arrows are incorrectly drawn and I drawn them correctly please check if it's right and write a little explanation how it's rightarrow_forwardAutoSave w | homework – Saved to my Mac OFF ... Home Insert Draw Design Layout References Mailings Review View O Tell me R Share O Comments Calibri (Bo. v 11 v A A E vE v E v E E Aa v AaBbCcDdEe AaBbCcD AaBbCcDdE AaBb AaBbCcDdEe Paste BIU V ab x, x A v I v A v No Spacing Normal Heading 1 Heading 2 Title Styles Pane Dictate Sensitivity Please answer the following questions fully and to the best of your ability 1) Please state how you would synthesize the following polymer below and show the mechanism behind the reaction. Note that this also means you need to tell me which molecule you are starting with as well as specifying all conditions required. он OH 2) Will either the polymer or the monomers you used to make it show peaks if IR spectroscopy analysis was done on them, and if so where? How about if UV-vis spectroscopy was performed instead? Page 1 of 1 93 words E English (United States) O Focus 白arrow_forward
- a laccd sign i X C tab os lock In McGraw-Hi X A ALEKS - Shi X McGraw-Hi x A ALEKS-Sh https://www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-IvUrTNdLZh5A8CnG03PBGuXr8iCPa7ZMmymzJtY9_DXbVcRRHtYf... All 0 O STATES OF MATTER Using Avogadro's Law esc K- Hydrogen gas and nitrogen gas react to form ammonia gas. What volume of ammonia would be produced by this reaction if 7.17 mL of nitrogen were consumed? Also, be sure your answer has a unit symbol, and is rounded to the correct number of significant digits. ALEKS Explanation 1 ? Type here to search A Z Mc Graw X 7 12 Check W S # 3 McGraw-Hi X A X alt E D $ 4 C 8 ロ・ロ R X O f5 ALEKS-Shu XMcGraw-Hi X % 5 Ai V T f6 G x10 6 17 4+ B & Y 7 H no 18 © 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | A a IAA * N J 19 M 1/5 K ho 86°F x x+ insert alt S prt sc 1arrow_forwarda laccd sign i X C tab McGraw-Hil X A ALEKS-Shu X https://www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-lgNslkr7j8P3jH-IvUrTNdLZh5A8CnG03PBGuXr8iCPa7ZMmym9HtY-pEBmXkvCfq... O CHEMICAL REACTIONS Calculating ion molarity using solute mass shift ↑ Calculate the molarity of Cl anions in the chemist's solution. Be sure your answer is rounded to the correct number of significant digits. caps lock esc Explanation ALEKS A chemist prepares a solution of vanadium(III) chloride (VC13) by measuring out 1.50 g of VCl₂ into a 300. mL volumetric flask and filling to the mark with distilled water. ILION K →1 E mol L ? Type here to search A Graw X HI Check 12 (8) 2 Z W S # 3 X alt $ D 4 C x10 f5 % X 100 LO 5 V f6 4- (0) 6 ALEKS G 14.1 f7 4+ & Y B hp 7 X H McGraw-Hi x A ALEKS-Shix Sign In 시기 fg IAA * © 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Center Accessibilit # a @ 76°F ㅅ 11:03 7/25/2 00 8 N 19 DII 9 fo K M DDI 2/5 0 P 12 insert [ = X + alt prt sc 46 Shusha 0 1 A backs paus ctearrow_forward||| = O MATTER Calculating volume by combining the volume of simple shapes A chemistry student in lab needs to fill a temperature-control tank with water. The tank measures 34.0 cm long by 17.0 cm wide by 14.0 cm deep. In addition, as shown in the sketch below, the student needs to allow 2.0 cm between the top of the tank and the top of the water, and a round-bottom flask with a diameter of 11.5 cm will be just barely submerged in the water. Calculate the volume of water in liters which the student needs. Round your answer to the nearest 0.01 L. Explanation Check 2 cm water X flask 21 OL m stv X 1/3 5 Kirste Ⓒ2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Acce Sall A O 20 9 Jarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY