
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:View
History
Bookmarks
Window
Help
Thu Mar 24 9:17 PM
A cvg.cengagenow.com
omewor..
BO Homewor
O PSY 220-.
O Chapter 6.
W Homewor.
* OWLV2 |-
O My Black
Documen
Sign in to
UMassD.
Extra Credit
[References)
et
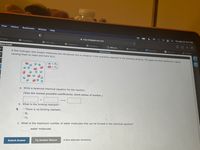
A few hydrogen and oxygen molecules are introduced into a container in the quantities depicted in the following drawing. The gases are then ignited by a spark,
causing them to react and form H2o.
- H,
- O,
a. Write a balanced chemical equation for the reaction.
(Use the lowest possible coefficients. Omit states of matter.)
b. What is the limiting reactant?
OThere is no limiting reactant.
O 02
C. What is the maximum number of water molecules that can be formed in the chemical reaction?
water molecules
8 item attempts remaining
Try Another Version
Submit Answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 11. Mrs. Parris demonstrates a reaction between a metal and a dissolved salt (aqueous). Balance the equation below and determine the mass of nickel produced when 15.4 grams of magnesium reacts with 20.0 grams of Ni(NO,), to produce Mg(NO,), Label the theoretical yield and limiting and excess reactant. (6.42 g Ni = ty, Ni(NO,), = LR) 3D Mg 15.4 grams _Ni(NO,), → 20.0 grams Ni + _Mg(NO,); +arrow_forwardReport Sheet: Exp. 15: PERIODIC PROPERTIES NOTE: All chemical equations must be balanced and must include physical states or phases. Name of partner: I. Reactivity with Water A. Lithium, Sodium and Potassium Describe the reaction of each metal with water. Include specific identification of the products formed. Na and H6₂0 Q-1 Q-2 feezing k and H₂0 feezing Li aindl H₂0 feezing Write the balanced chemical equation for each reaction: Q-3 and Q-4 turn smoke andl Pink ignition turn Pink Clear Rank:arrow_forwardII AM Lab data percent yield carbon dioxide.pdf Name Sect Percent Yield of Carbon Dioxide To predict the amount of carbon dioxide gas that should be produced in a Purpose: chemical reaction. Then calculate the amount of carbon dioxide actually produced and determine the percent yield. The reaction is represented in the equation below: CH,COOH + NaHCO, → NaCH,C00 + H20 + CO2 Materials: sodium bicarbonate (aka baking soda), acetic acid solution (aka vinegar), Erlenmeyer flasks, balance .Procedure: 1. Measure and record the mass of a 250-mL Erlenmeyer flask. Label it as flask A. 2. Add approximately 7.5 g of sodium bicarbonate to flask A. 3. Measure and record the mass of a second 250-ml Erlenmeyer flask. Call it flask B. 4. Add approximately 75 g of acetic acid solution to flask B. 5. Slowly add acetic acid to flask A until the reaction has stopped. Do not add all of the vinegar; just enough to complete the reaction. 6. After the reaction has stopped, measure and record the masses of…arrow_forward
- [References] TUTOR Chemical Equations: Gram/Gram Calculation Consider the reaction: SOe) + H;O(1) → H3SO;( Given an initial mass of 15.3 g SO, an excess of H50, and assuming that all of the reactant is converted to product(s), and none is lost, calculate the mass (g) of H7SO3 produced by the reaction.arrow_forwardPlease refer to the image i need this done asap pleaseeee show the workarrow_forwardIn the earth's primitive atmosphere, it is thought that water could have reacted with methane to produce hydrogen gas and carbon monoxide. CH(g) + H;O(g) → CO(g)+ 3H2(g) The particulate representation to the right shows a product mixture for the hydrogen forming reaction above. Which species is the limiting reactant? A. CH,(g) В. Н.О(g) C. Co(g) D. H,(g) E. It is a stoichiometric mixturearrow_forward
- the reaction of 15 moles of carbon with 30 moles of oxygen will result in a theoretical yield of ____ moles of CO2 is what?arrow_forwardFor the sentence below answer the following questions: Q1) Change the sentence into a chemical equation Q2) What are the reactants and products? "1 mole of aqueous Pb(NO3)2 reacts with 2 moles of aqueous KI to produce 1 mole of solid Pbl2 and 2 moles of aqueous KNO3." в I U Format ... >arrow_forward2. Octane (a common organic solvent, and a major component of gasoline, mol formula: C8H18) burns in the presence of oxygen. The combustion produces carbon dioxide, water, and heat. If we burn 325.2 g of octane, determine: a. The balanced chemical equation for the combustion reaction (you can omit heat) b. The mass of oxygen consumed in the reactionarrow_forward
- Please do this, they are all parts of 1 question do all please.arrow_forward3. Consider the following balanced reaction: 2 Fe + 3 Cl₂ → 2 FeCl3 Given 60.0 g of Fe and excess Cl₂ (as much as we need), calculate: a. The theoretical yield of FeCl3, in grams. b. The theoretical amount of Cl₂ consumed, in grams.arrow_forward4. A mixture of KC1O3 and MnO2 weighing 1.75g was heated to 3000C until decomposition was complete. After cooling the mixture weighed 1.11 g. a) write the balance equation of the decomposition. b) how many grams and moles of 02 were produced in the decomposition?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY