
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
What is the volumetric flow rate in L/h of Feed in the attached picture?
converting to the volume of the feed, show all steps, and be professional.
Transcribed Image Text:The use
All
e.
c. For
a prox
blems,
tional
sare
3 lists
Those
need
r the
aran-
that
im-
-h as
nvenient
product degradation or undesirable agglomeration, a pilot-
plant is necessary. Operations near the middle usually require
laboratory data, while those near the bottom require pilot-
plant tests.
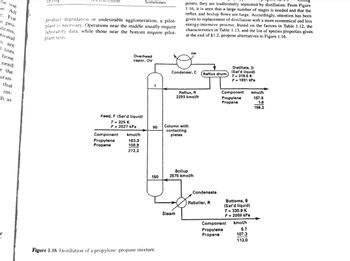
Feed, F (Sat'd liquid)
T = 325 K
P = 2027 kPa
Sometimes
Overhead
vapor, OV
Component kmol/h
163.3
108.9
272.2
Propylene
Propane
1
90
150
Figure 1.16 Distillation of a propylene-propane mixture.
points, they are traditionally separated by distillation. From Figure
1.16, it is seen that a large number of stages is needed and that the
reflux and boilup flows are large. Accordingly, attention has been
given to replacement of distillation with a more economical and less
energy-intensive process. Based on the factors in Table 1.12, the
characteristics in Table 1.13, and the list of species properties given
at the end of $1.2, propose alternatives to Figure 1.16.
Condenser, C
CW
Column with
contacting
plates
Steam
Reflux, R
2293 kmol/h
Boilup
2575 kmol/h
(Reflux drum
Condensate
Reboiler, R
Distillate, D
(Sat'd liquid)
T-319.5 K
P = 1931 kPa
Component
Propylene
Propane
Bottoms, B
(Sat'd liquid)
T-330.9 K
P=2069 kPa
Component
Propylene
Propane
kmol/h
5.7
107.3
113.0
kmol/h
157.6
1.6
159.2
Expert Solution
arrow_forward
Step 1
Given:
The molar flow rate of the feed (propylene and propane) is given to us. We require the volume flow rate (in L/h) of the feed.
To calculate the volumetric flow rate, we need two quantities, the mass flow rate and the density of the substance, since the volume flow rate is given as follows:
So, we need to calculate the density and the mass flow rate for both components first.
Step by stepSolved in 4 steps

Knowledge Booster
Similar questions
- Back HW#2 QUESTION1/60pts] A fluid of viscosity u flows in the horizontal cylinder (radius R) shown in the figure under a constant pressure gradient dP/dx. The inner core of the cylinder is filled with a porous material. The flow in this porous region is slow and assumed to be a plug-type flow such that the velocity is constant and everywhere the same inside the porous region. Denote this velocity by Uo. The flow in the open (non-porous) region is steady, Newtonian, incompressible and axisymmetric. It will be assumed that only the axial (x) component of the velocity is non-zero. Flow Open flow T&R Porous media flow R N.B. All your answers must be expressed in terms of µ, U₁, a, R and dP/dx. (a) Use the continuity and Navier-Stokes equations to determine the expression of the velocity in the open region. (b) What is the expression of the average velocity in the open region? (c) Is the assumption of a linear velocity profile in the open region acceptable when this region Tuatifi 1:arrow_forwardA ventilation opening in a room is 2 feet by 3 feet. Air flow out of the opening is measured at 75 feet per minute. Calculate the CFM rate.arrow_forwardWhat do you mean by volumetric flow rate?arrow_forward
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The