
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Could someone please help I know it agaisnt the guidelines but please!
No plagiarism, please!
LeChatelier’s principle states, “When a change is made to an equilibrium system, the system moves to counteract the imposed change and restore the system to equilibrium.” Use your observations from part 2 to answer the following questions:
- Explain in terms of LeChatelier’s principle the observations made when KSCN was added to the system (Was the forward or reverse reaction favored?
- Explain in terms of LeChatelier’s principle the observations made when NaCl was added to the system (Was the forward or reverse reaction favored? )
- Explain in terms of LeChatelier’s principle the observations made when silver nitrate was added to the system(Was the forward or reverse reaction favored?)
- Explain in terms of LeChatelier’s principle the observations made when Fe(NO3)3 was added to the system(Was the forward or reverse reaction favored? What happened to the reactants or products as a result of adding ?).
- Explain in terms of LeChatelier’s principle and your observations upon the addition of heat to and removal of heat from the system (Was this reaction endothermic or exothermic? Was heat a reactant or product of this reaction?).
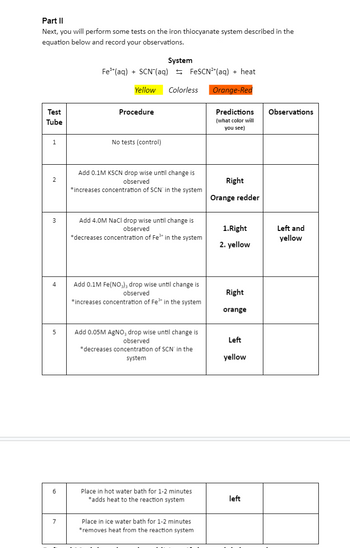
Transcribed Image Text:Part II
Next, you will perform some tests on the iron thiocyanate system described in the
equation below and record your observations.
Test
Tube
1
2
3
4
5
6
7
Fe³+ (aq) + SCN (aq)
System
Procedure
Yellow Colorless
No tests (control)
FeSCN²(aq) + heat
Orange-Red
Add 0.1M KSCN drop wise until change is
observed
*increases concentration of SCN in the system
Add 4.0M NaCl drop wise until change is
observed
*decreases concentration of Fe³+ in the system
Add 0.1M Fe(NO3)3 drop wise until change is
observed
*increases concentration of Fe³+ in the system
Add 0.05M AgNO, drop wise until change is
observed
*decreases concentration of SCN' in the
system
Place in hot water bath for 1-2 minutes
*adds heat to the reaction system
Place in ice water bath for 1-2 minutes
*removes heat from the reaction system
Predictions
(what color will
you see)
Right
Orange redder
1. Right
2. yellow
Right
orange
Left
yellow
left
Observations
Left and
yellow
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The Ins and Outs of Équilibrium If you leave a closed, partly filled bottle of water in the sunlight, before long you will observe water droplets near the top of the bottle and in the neck. How did they get there? As the sun shines on the bottle, the water begins to evaporate. As the number of vapor molecules increases, so does the chance that they will interact with each other and recondense to form water. This is how the water droplets get to the top of the bottle. Changing phase is a reversible process. In a closed container, as the amount of vapor increases and the amount of liquid decreases, the rate of condensation increases and the rate of vaporization decreases. Eventually the two rates become equal. When the rate of vaporization is equal to the rate of condensation, the amount of vapor and the amount of liquid stops changing. This is called equilibrium. Just because the rate of vaporization and condensation is equal at equilibrium, it doesn't mean that the amount of vapor and…arrow_forwardnot graded!arrow_forwardFor the reaction system Fe(s) + H2 O(g)= FeO(s) + H2 (9) which has already reached a state of equilibrium, predict the effect that each of the following changes will have on the position of the equilibrium. Tell whether the equilibrium will shift to the right, will shift to the left, or will not be affected. a The pressure of hydrogen is increased by injecting an additional mole of hydrogen gas into the reaction vessel. shifts left shifts right no effect b Hydrogen gas is removed as it forms by use of a chemical absorbent, or "scrubber." shifts left shifts right no effect C An additional amount of solid iron is added to the reaction vessel. shifts left shifts right no effectarrow_forward
- Given the reaction: CO(NH2),(s) + H2O(g) = CO2(9) + 2NH3(g) -- Kp If this reaction system is initially at equilibrium and a catalyst is added, the new position of equilibrium will be = 1.39 at 500 K The value of K, for the given reaction at 600 K is The reaction CO(NH₂)₂(s) + H₂O(g) → CO₂(g) + 2 NH₂(g) is -- A flask containing CO₂(g), NH₂(g), CO(NH₂)₂(s) and H₂O(g) is initially at equilibrium at 500 K. When the flask is heated to 600 K, the amounts of CO(NH₂)₂(s) and H₂O(g) decrease and the amounts of CO₂(g) and NH₂(g) increase. This means that:arrow_forwardConsider the reaction of SO2 and O2 described by the chemical reaction below. Determine the equilibrium constant for this reaction by constructing an ICE table, writing the equilibrium constant expression, and solving it. Complete Parts 1-2 before submitting your answer. NEXT > A 2.00 L reaction vessel was filled 0.0432 mol SO2 and 0.0296 mol O2 at 900 K and allowed to react. At equilibrium, the concentration of SO3 was found to be 0.0175 M. Fill in the ICE table with the appropriate value for each involved species to determine concentrations of all reactants and products. Initial (M) Change (M) Equilibrium (M) -0.00875 0.0209 0 0.00875 2 SO₂(g) + O₂(g) = 2 SO³(g) 2SO2(g) 2.00 0.0216 0.0432 0.0148 + 0.0296 0.0041 O₂(g) 2 0.0175 0.0129 -0.0175 0.0061 2SO3(g) RESET -0.0350 0.0257arrow_forwardConsider the reaction: N₂O4 (9) — 2NO2 (9) Write the equilibrium constant for this reaction in terms of the equilibrium constants, K₁ and K2, for the reactions below: N₂(g) +202 (g) = N₂O4 (9) K₁ 1/2N₂(g) + O2(g) — NO2(g) K₂ For answers with both a subscript and a superscript, enter the subscript first. For example, enter Kif the first equilibrium constant should be squared. K=arrow_forward
- Consider the reaction of N₂O and O2 described by the chemical reaction below. Determine the equilibrium constant for this reaction by constructing an ICE table, writing the equilibrium constant expression, and solving it. Complete Parts 1-2 before submitting your answer. NEXT > A 1.00 L reaction vessel was filled with 0.0560 mol O₂ and 0.200 mol N₂O and allowed to react at 298 K. At equilibrium, there were 0.0200 mol of NO2 present. Fill in the ICE table with the appropriate value for each involved species to determine concentrations of all reactants and products.. Initial (M) Change (M) Equilibrium (M) 0.0100 0.0410 0 -0.0100 0.0710 1 2 N₂O(g) + 3 O₂(g) = 4 NO2(g) 2N₂O(g) 0.0560 -0.0050 0.0360 0.200 0.0050 0.180 + -1.00 0.0150 30₂(g) 2 -0.0560 -0.0150 0.0200 0.210 4NO₂(g) RESET -0.0200 0.190 +arrow_forwardPredict and calculate the effect of concentration changes on an equilibrium system. Some (CH3)2CHOH is allowed to dissociate into (CH3)2CO and H₂ at 452 K. At equilibrium, [(CH3)2CHOH] = 0.294 and [(CH3)₂CO] = [H₂] = 5.94x10-2 M. Additional (CH3)2CO is added so that [(CH3)2CO]new = 9.02x10-2 M and the system is allowed to once again reach equilibrium. (CH3)2CHOH(g)(CH3)2CO(g) + H₂(9) (a) In which direction will the reaction proceed to reach equilibrium? (b) What are the new concentrations of reactants and products after the system reaches equilibrium? [(CH3)2CHOH] = [(CH3)2CO] = [H₂] M M M = 1.20x10-2 at 452 Karrow_forwardPlease answer the following questions attached below. Follow the instructions for each given item and provide the complete solution to help me understand the topic. Thank you! P.S. Please do make sure the answers are correct for this items as I only have a few questions left.arrow_forward
- Q1) For the equilibrium: A B, if [A].0.569, [B].0.162, and Kea 47.8, what are [A]es and [B]ea? algebraic expression: [A]eq= Q2) For the equilibrium: 1221, if [2]. 1.26, [1].0, and Keq = 1.14 x10, what are [12]eg and [lleg? E algebraic expression: [H₂leq_ [12]eg [B]ea. algebraic expression: [12]ea. [eq M Q3) For the equilibrium: H₂ + 1₂2H1, if [H₂lo=0.546, [b]o 1.26, [HI].0, and Kea 45.3, what are [Halea, [lzles and [HI]eq? =0 [HI] eq = 0 0arrow_forwardInitial (M) Change (M) Equilibrium (M) Consider the reaction of SO₂ and O₂ described by the chemical reaction below. Determine the equilibrium constant for this reaction by constructing an ICE table, writing the equilibrium constant expression, and solving it. Complete Parts 1-2 before submitting your answer. 2 SO₂(g) + O₂(g) = 2 SO₂(g) NEXT A 2.00 L reaction vessel was filled 0.0432 mol SO₂ and 0.0296 mol O₂ at 900 K and allowed to react. At equilibrium, the concentration of SO, was found to be 0.0175 M. Fill in the ICE table with the appropriate value for each involved species to determine concentrations of all reactants and products. -0.00875 0.0209 0 0.00875 2SO₂(g) Question 20 of 33 2.00 0.0216 0.0432 0.0148 0.0296 0.0041 O₂(g) 0.0175 2 0.0129 -0.0175 0.0061 RESET -0.0350 0.0257 2SO₂(g)arrow_forwardHydrogen and chlorine react to form hydrogen chloride, like this: H,(g) + Cl,(g) 2 HCl(g) Also, a chemist finds that at a certain temperature the equilibrium mixture of hydrogen, chlorine, and hydrogen chloride has the following composition: compound concentration at equilibrium 0.86 M Cl, 0.30 M HC1 1.9 M Calculate the value of the equilibrium constant K for this reaction. Round your answer to 2 significant digits. K = 0arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY