
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
draw the starting structure that would lead to this major product (and its enantiomer) under these conditions
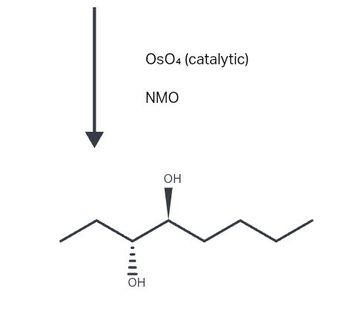
Transcribed Image Text:OH
OsO4 (catalytic)
NMO
OH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
this solution is incorrect, please help
Solution
by Bartleby Expert
Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
this solution is incorrect, please help
Solution
by Bartleby Expert
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw the four major products that form, including stereoisomers, in the bromination of cyclopentene with Br2 and light. Make sure you draw all four bonds at all stereocenters. Br₂ hyarrow_forwardDraw the starting alkene that would lead to this the major product (and its enantiomer) under these conditions. Drawing H2 Pd/C OD0 000 F5 F4arrow_forward5. Which bromocyclohexane starting material would react faster - the cis or trans, and explain why? Draw both chair forms of the starting material to prove your point. Br K* OC(CH) K* OC(CH); Br cis transarrow_forward
- 2. What products would be obtained by treating A-D with NaOt-Bu in t-BuOH? Explain your reasoning, and if more than one product forms, indicate which product is major. Br A Br B C لي Br Darrow_forwardDraw How this molecule reacts with Br2 Then identify halogenated product Then in mono halogenation product, determine r/s configuration as well as labeling products being chiral or achiralarrow_forwardFor the following reaction, decide whether a solution of the products would be optically active or not and why. You will need to know the product of this reaction in order to solve this problem. To preview the image click here Br CH3 Na: CEN: acetone O Not optically active, achiral product is formed O Not optically active, racemic mixture is formed O Not optically active, a meso compound is formed O Optically active, a single enantiomer is formedarrow_forward
- Draw all of the products, both constitutional isomers and stereoisomers, of the following reactions. a) 4,4-dibromo-1-ethylcyclopentene ---->Br2,H2O b) 3,3-dibromo-1-ethylcyclopentene ----> HBr c) 3,3-dibromo-1,2-dimethylcyclopentene ----> 1. BH3. 2. H2O2, NaOHarrow_forwardNonearrow_forwardPredict the stereochemistry for the following E2 reaction. Draw a Newmann Projection of the reactive conformation and the structure for only major product of the reactionarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY