
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
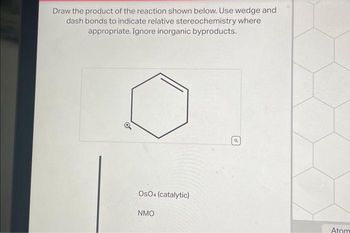
Transcribed Image Text:Draw the product of the reaction shown below. Use wedge and
dash bonds to indicate relative stereochemistry where
appropriate. Ignore inorganic byproducts.
OSO4 (catalytic)
NMO
a
Atom
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Complete the balanced molecular chemicahal equation for the reaction below. If no reaction occurs, write NR after the reaction arrow. RB2CO(aq)+Fe(C2H3)2)2(aq)> Please explain Thanks.arrow_forwardNH2 CH, \ O O N- phosphorus anhydride linkage H;CCSCH,CH,NHCCH,CH,NHËCHCCH,OPOPOCH, HO CH, HO OH N N. acetyl group phosphate monoester group ОН amide group О—Р— О- O |曲 75°F Sunny EOarrow_forwardGiven each value, determine whether the starting material or product isfavored at equilibrium.a.) Keq = 0.5b.) ΔGo = −100 kJ/molc.) ΔHo = 8.0 kJ/mold.) Keq = 16e.) ΔGo = 2.0 kJ/molf.) ΔHo = 200 kJ/molg.) ΔSo = 8 J/(K•mol)h.) ΔSo = −8 J/(K•mol)arrow_forward
- What type of reaction is shown below? PbSO4(aq) + 2HCl(aq) ==> PbCl2(s) + H2SO4(aq) What is produced?arrow_forward3. According to the Hammond Postulate, what does transition state 3 (TS3) most closely resemble? First choice: Second choice: AG (a) TS2 (b) P (c) 12 (d) 11 TS2 TS1 M 11 Reaction Coordinate 12 TS3arrow_forward2. Give the products of the reactions below H₂(g), Pd/C H₂(g), PtO2, KMnO4arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY