
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
The orbital diagram that follows presents the final step in the
formation of hybrid orbitals by a silicon atom. (a) Which of
the following best describes what took place before the step
pictured in the diagram: (i) Two 3p electrons became unpaired,
(ii) An electron was promoted from the 2p orbital to
the 3s orbital, or (iii) An electron was promoted from the 3s
orbital to the 3p orbital? (b) What type of hybrid orbital is
produced in this hybridization? [
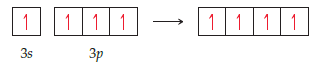
Transcribed Image Text:|1111
1111
3s
Зр
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- . Give the hybridization and oxidation state for sulfur in SO2, in SO3, and in H2SO4. And draw the Lewis structures to determine the hybridizationarrow_forwardWhat is the hybridization the central atom in the structure shown below? :0=Br-O: :Ö: →arrow_forwardWhat is the hybridization the central atom in the structure shown below?arrow_forward
- What is the hybridization of the central atom in the periodate (10,) anion?arrow_forward5. (1) Describe the difference of hybrid orbitals such as sp, sp? and sp3. (2) Why diamond and the lead of a pencil show different electrical properties with same composition by carbon atom?.arrow_forwardFor each of the following molecules, indicate the hybridization: (a) carbon dioxide (CO2) central O hybridization (b) formaldehyde (COH2) central C hybridization (c) phosphorous dioxide (PO2) central P hybridization (d) phosphate ion (PO43-) central P hybridizationarrow_forward
- Which molecule has a trigonal pyramidal geometry and the central atom has the sp3 hybridization? (A) BCl3 (B) BrCl3 (C) PCl3 (D) All of themarrow_forward(a) Using the molecular orbital model, write the valence electron configuration for CN and use it to calculate the bond order. Is the species paramagnetic? Bond order = Paramagnetic? (b) Using the molecular orbital model, write the valence electron configuration for CO and use it to calculate the bond order. Is the species paramagnetic? Bond order = Paramagnetic?arrow_forwardValence bond theory The skeletal structure for methyleneimine (CH₂NH) is shown. Draw for yourself the best Lewis structure. Propose a bonding scheme by indicating the hybridization of the central atoms and the orbital overlaps for each bond. (a) H one (b) H-C-N-H The bond labeled (a) forms from The bond labeled (b) forms from: ● one o-overlap of a C (c) π-overlap (s) of a C -overlap of a C sp2 orbital and a N orbital and a N orbital and a H 1s The ideal bond angle <(C-N-H) around the N atom is orbital, and orbital. The bond labeled (c) forms from O - overlap of a N There is/are one lone pair(s) around the N atom. Lewis structures do not attempt to portray 3D shape, but you can predict the molecular geometry from VSEPR theory. The ideal bond angle <(H-C-H) around the C atom is 120 orbital and a H 1s degrees. orbital. degrees. orbital.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY