
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
![### Writing Equilibrium Equations for Reactions
This section guides you through writing the equilibrium constant expression (\( K \)) for several chemical reactions.
#### Part A
**Reaction:**
\[ \text{S}_2(g) + 2\text{H}_2(g) \rightleftharpoons 2\text{H}_2\text{S}(g) \]
**Options for Equilibrium Constant (\( K \)):**
1. \( K = \frac{[\text{H}_2\text{S}]^2}{[\text{S}_2][\text{H}_2]^2} \)
2. \( K = \frac{[\text{H}_2\text{S}]}{[\text{S}_2][\text{H}_2]} \)
3. \( K = \frac{[\text{H}_2\text{S}]^2}{[\text{S}_2]^2} \)
4. \( K = \frac{[\text{H}_2\text{S}]}{[\text{S}_2][\text{H}_2]^2} \)
#### Part B
**Reaction:**
\[ \text{H}_2\text{S}(aq) + \text{Cl}_2(aq) \rightleftharpoons \text{S}(s) + 2\text{HCl}(aq) \]
**Options for Equilibrium Constant (\( K \)):**
1. \( K = \frac{[\text{HCl}]^2}{[\text{Cl}_2][\text{H}_2\text{S}]} \)
2. \( K = \frac{[\text{Cl}_2][\text{H}_2\text{S}]}{[\text{HCl}]} \)
3. \( K = \frac{[\text{HCl}]}{[\text{Cl}_2]^2[\text{H}_2\text{S}]} \)
#### Part C
**Reaction:**
\[ \text{Br}_2(g) + \text{Cl}_2(g) \rightleftharpoons 2\text{BrCl}(g) \]
**Options for Equilibrium Constant (\( K \)):**
1. \( K = \frac{[\text{BrCl}]^2}{[\text](https://content.bartleby.com/qna-images/question/7f185ec2-8c2e-4aee-a8f1-0a1f41f71cb3/38b72ee9-6ec5-412b-87dd-ae94add7754e/viw7au_thumbnail.jpeg)
Transcribed Image Text:### Writing Equilibrium Equations for Reactions
This section guides you through writing the equilibrium constant expression (\( K \)) for several chemical reactions.
#### Part A
**Reaction:**
\[ \text{S}_2(g) + 2\text{H}_2(g) \rightleftharpoons 2\text{H}_2\text{S}(g) \]
**Options for Equilibrium Constant (\( K \)):**
1. \( K = \frac{[\text{H}_2\text{S}]^2}{[\text{S}_2][\text{H}_2]^2} \)
2. \( K = \frac{[\text{H}_2\text{S}]}{[\text{S}_2][\text{H}_2]} \)
3. \( K = \frac{[\text{H}_2\text{S}]^2}{[\text{S}_2]^2} \)
4. \( K = \frac{[\text{H}_2\text{S}]}{[\text{S}_2][\text{H}_2]^2} \)
#### Part B
**Reaction:**
\[ \text{H}_2\text{S}(aq) + \text{Cl}_2(aq) \rightleftharpoons \text{S}(s) + 2\text{HCl}(aq) \]
**Options for Equilibrium Constant (\( K \)):**
1. \( K = \frac{[\text{HCl}]^2}{[\text{Cl}_2][\text{H}_2\text{S}]} \)
2. \( K = \frac{[\text{Cl}_2][\text{H}_2\text{S}]}{[\text{HCl}]} \)
3. \( K = \frac{[\text{HCl}]}{[\text{Cl}_2]^2[\text{H}_2\text{S}]} \)
#### Part C
**Reaction:**
\[ \text{Br}_2(g) + \text{Cl}_2(g) \rightleftharpoons 2\text{BrCl}(g) \]
**Options for Equilibrium Constant (\( K \)):**
1. \( K = \frac{[\text{BrCl}]^2}{[\text
![**Write the equilibrium equations for the following reactions:**
### Part B
**Reaction:**
\[ \text{H}_2\text{S(aq)} + \text{Cl}_2\text{(aq)} \rightleftharpoons \text{S(s)} + 2\text{HCl(aq)} \]
**Equilibrium Expressions:**
- \( K = \frac{[\text{HCl}]^2}{[\text{Cl}_2][\text{H}_2\text{S}]} \)
- \( K = \frac{[\text{S}][\text{H}_2\text{S}]^2}{[\text{HCl}]^2} \)
- \( K = \frac{[\text{Cl}_2][\text{H}_2\text{S}]}{[\text{HCl}]} \)
- \( K = \frac{[\text{HCl}]}{[\text{Cl}_2][\text{H}_2\text{S}]} \)
**Options to select from are formatted as bulleted lists with different equilibrium constant (K) expressions.**
---
### Part C
**Reaction:**
\[ \text{Br}_2\text{(g)} + \text{Cl}_2\text{(g)} \rightleftharpoons 2\text{BrCl(g)} \]
**Equilibrium Expressions:**
- \( K = \frac{[\text{BrCl}]^2}{[\text{Br}_2][\text{Cl}_2]} \)
- \( K = \frac{[\text{BrCl}]}{[\text{Br}_2][\text{Cl}_2]^2} \)
- \( K = \frac{[\text{BrCl}]}{[\text{Br}_2]\left[\text{Cl}_2\right]^{2}} \)
- \( K = \frac{[\text{BrCl}]^2}{[\text{Br}_2][\text{Cl}_2]} \)
**The options are demonstrated with different ways of expressing the equilibrium constant (K) calculations.**
---
### Part D
**Reaction:**
\[ \text{C(s)} + \text{H}_2\text{O(g)} \rightleftharpoons \text{CO(g)} + \text](https://content.bartleby.com/qna-images/question/7f185ec2-8c2e-4aee-a8f1-0a1f41f71cb3/38b72ee9-6ec5-412b-87dd-ae94add7754e/evhlvs_thumbnail.jpeg)
Transcribed Image Text:**Write the equilibrium equations for the following reactions:**
### Part B
**Reaction:**
\[ \text{H}_2\text{S(aq)} + \text{Cl}_2\text{(aq)} \rightleftharpoons \text{S(s)} + 2\text{HCl(aq)} \]
**Equilibrium Expressions:**
- \( K = \frac{[\text{HCl}]^2}{[\text{Cl}_2][\text{H}_2\text{S}]} \)
- \( K = \frac{[\text{S}][\text{H}_2\text{S}]^2}{[\text{HCl}]^2} \)
- \( K = \frac{[\text{Cl}_2][\text{H}_2\text{S}]}{[\text{HCl}]} \)
- \( K = \frac{[\text{HCl}]}{[\text{Cl}_2][\text{H}_2\text{S}]} \)
**Options to select from are formatted as bulleted lists with different equilibrium constant (K) expressions.**
---
### Part C
**Reaction:**
\[ \text{Br}_2\text{(g)} + \text{Cl}_2\text{(g)} \rightleftharpoons 2\text{BrCl(g)} \]
**Equilibrium Expressions:**
- \( K = \frac{[\text{BrCl}]^2}{[\text{Br}_2][\text{Cl}_2]} \)
- \( K = \frac{[\text{BrCl}]}{[\text{Br}_2][\text{Cl}_2]^2} \)
- \( K = \frac{[\text{BrCl}]}{[\text{Br}_2]\left[\text{Cl}_2\right]^{2}} \)
- \( K = \frac{[\text{BrCl}]^2}{[\text{Br}_2][\text{Cl}_2]} \)
**The options are demonstrated with different ways of expressing the equilibrium constant (K) calculations.**
---
### Part D
**Reaction:**
\[ \text{C(s)} + \text{H}_2\text{O(g)} \rightleftharpoons \text{CO(g)} + \text
Expert Solution
arrow_forward
Step 1
Equilibrium constant expression:
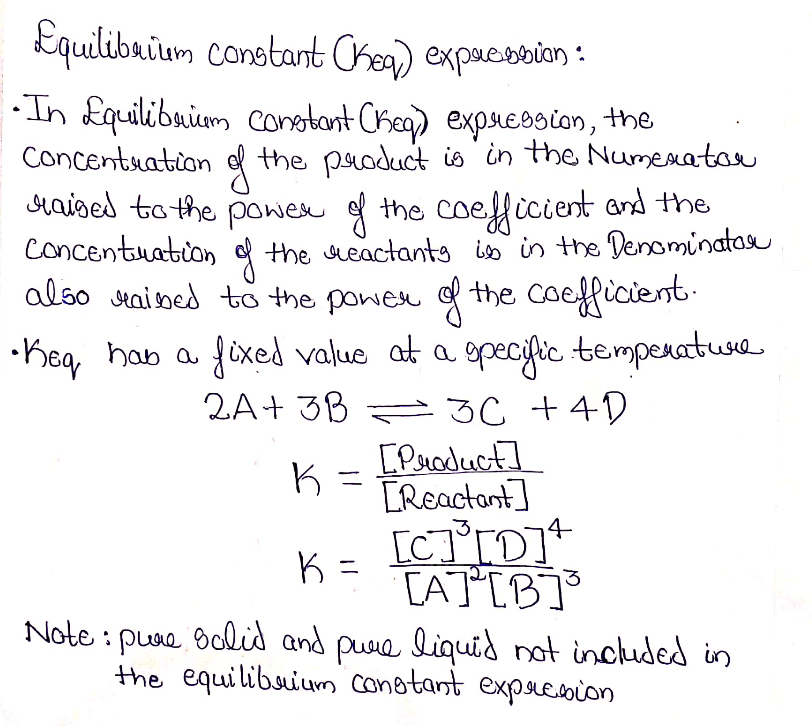
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Complete and balance the equations for the following reactions performed in the wells. Be sure to include states of matter for your reactants and products. Write “NR” if there is no reaction. BaCl2 + Na2SO4 CaCl2 + Na2CO3 MgCl2 + (NH4)2C2O4 SrCl2 + KIO3arrow_forwardA mixture of butene, C4H8, and butane, C4H10, is burned in air to give CO2 and water. Suppose you burn 2.85 g of the mixture and obtain 8.75 g of CO2 and 4.13 g of H₂O. What are the mass percentages of butene and butane in the mixture? Mass percentage of butene = Mass percentage of butane = % %arrow_forward3. For each acid, name the anion bonded to hydrogen, then name the acid. Anion Name Acid Name (а) НBr (b) НF (c) H;S (d) HNO; (e) HC10;arrow_forward
- If 465 g of Pb(NO3)2 (FW 331.21 g/mol) is added to a solution containing 215 g of K3PO4 (FW 212.27 g/mol), what mass, in g, of Pb3(PO4)2 (FW 811.54 g/mol) precipitate will be formed? The balanced equation is 3 Pb(NO3)2(aq) + 2 K3PO4(aq) → Pb3(PO4)2(S) + 6 KNO3(aq)arrow_forward[Review Topics] [References] Use the References to access important values if needed for this question. For the following reaction, 136 grams of perchloric acid (HCIO4) are allowed to react with 25.6 grams of tetraphosphorus decaoxide. perchloric acid (HClO4) (aq) + tetraphosphorus decaoxide(s) → phosphoric acid (aq) + dichlorine heptaoxide (1) What is the maximum amount of phosphoric acid that can be formed? Mass= g What is the FORMULA for the limiting reactant? What amount of the excess reactant remains after the reaction is complete? Mass= g Submit Answer Retry Entire Group Show Hint 9 more group attempts remaining Previous Next> Save and Exitarrow_forwardAntimony is alloyed with lead to increase the rigidity of components used in the construction of lead storage batteries.1.304 g of a particular metallic alloy, compounded of only Pb and Sb, can be quantitatively converted into a 1.511-g mixture of the oxides PbO2 and Sb2O4.What was the percentage (by mass) of antimony in the alloy?arrow_forward
- Which reaction has the largest AS? ON₂(g) + 3H₂(g) → 2NH3(9) □2NO(g) → N₂O2(g) O2N₂ H₁(g) →→→ 2NH3(g) + H₂(g) □ O₂(g) + 2H₂(g) → 2H₂O(g) □ O2(g) +2H₂(g) → 2H₂O(l)arrow_forwardA student combines an aqueous solution of Fe(NO3)2 with an aqueous solution of sodium carbonate; a precipitate forms. What ions form the precipitate in this reaction? Fe2+ and CO32- Nat and Fe2+ Fe2+ and NO3 CO32 and NO3 O Na* and NO3arrow_forwardWhat mass of Cu(IO3)2 can be formed from 0.650 g of CuSO4 · 5H2O? What mass of KIO3 is needed to convert the copper in 0.2750 g of CUSO4 - 5H2O to Cu(IO3)2?arrow_forward
- Phosgene, a substance used in poisonous gas warfare during World War I, is so named because it was first prepared by theaction of sunlight on a mixture of carbon monoxide and chlorine gases. Its name comes from the Greek words phos (light) andgenes (born of). Phosgene has the following elemental composition: 12.14% C, 16.17% O, and 71.69% Cl by mass. Its molarmass is 98.9 g>mol. (a) Determine the molecular formula of this compound. (b) Draw three Lewis structures for the moleculethat satisfy the octet rule for each atom. (The Cl and O atoms bond to C.) (c) Using formal charges, determine which Lewisstructure is the dominant one. (d) Using average bond enthalpies, estimate ΔH for the formation of gaseous phosgene fromCO(g) and Cl21g2.arrow_forward10. Write the completed and balanced equation with the correct physical state for each species in molecular, ionic and net ionic form. CS3PO4 (aq) + Ge(C2H3O2)4 (aq) → lonic: Net lonic:arrow_forwardClassify each chemical reaction: NaOH(aq) + HBrO (aq) → NaBrO (aq) + H₂O(1) Ca (s) + Br₂ (1) CaBr₂ (s) reaction CH₂CH₂CH, (g) + 50₂(g) 2 CH₂ (CH₂), CH₂ (8) + 802₂ (8) 3 3CO₂ (g) + 4H₂O (g) 2 5CO₂(g) + 6H₂0(g) type of reaction (check all that apply) combination single replacement double replacement decomposition combination single replacement double replacement decomposition combination single replacement double replacement decomposition combination single replacement double replacement decomposition precipitation combustion acid-base precipitation combustion acid-base precipitation combustion acid-base precipitation combustion acid-basearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY