
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
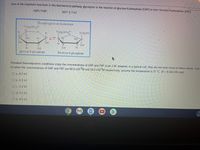
Transcribed Image Text:One of the important reactions in the biochemical pathway glycolysis is the reaction of glucose-6-phosphate (G6P) to form fructose-6-phosphate (F6P):
G6P F6P
AG-1.7 kJ
Phosphoglucose Isomerase
6 CH2OPO,
2-
H.
CH2OPO3
ICH,OH
H.
H.
HO.
H.
1.
H.
HO.
HO.
H.
14
B OH
H.
H.
HO.
gluco se-6-phosphate
fructo se-6-phosphate
Standard thermodynamic conditions imply the concentrations of G6P and F6P to be 1 M, however, in a typical cell, they are not even close to these values. Calc
kJ when the concentrations of G6P and F6P are 60.0 ×10M and 14.0 x10 M respectively, assume the temperature is 37 °C. (R = 8.314 J/K-mol)
O a. -0.1 kJ
Ob.-1.1 kJ
Oc.-2.1 kJ
O d.-3.1 kJ
O e.-4.1 kJ
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Part A Which of the following is a product in the following reaction? glucose + fructose sucrose + H₂O sucrose fructose oxygen (O₂) glucose Submit My Answers Give Up Part B Examine the following reactions. Which of the following is true about compound C? Reaction 1 Reaction 2 Reaction 3 Reaction 4 G+H A+B → Submit C + D → E+F→ C is a product for reaction 2 only. C is the product of reaction 1 and a reactant for reaction 2. C is the reactant of reaction 1 and a product for reaction 2. C is a reactant for reaction 1 only. My Answers Give Uparrow_forwardN-NHPH CHO CH2OH H- C=N-NHPH Но- -H- 3 equiv Но- -H- 3 equiv Но- PHNHNH2 PHNHNH, H- H- OH H- -O- H- H- -HO- H- -HO- ČH2OH ČH2OH ČH2OH D-glucose osazone D-fructose + NH3 + PHNH2 + 2 H20 The final step in the formation of the osazone from glucose is the reaction of the keto imine with 2 equivalents of phenylhydrazine to yield the osazone plus ammonia. This reaction involves the following intermediate steps: 1. Addition of phenylhydrazine to the imine and proton transfer to yield intermediate 1; 2. Elimination of ammonia to yield phenylhydrazone 2; 3. Addition of phenylhydrazine to the ketone to yield tetrahedral intermediate 3; 4. Proton transfer yields carbinolamine 4; 5. Elimination of water yields the final product osazone. Write out the mechanism on a separate sh of paper and then draw the structure of tetrahedral intermediate 3. NH НО- -H H- -ОН H- -ОН ОН Glucose keto imine Previous Nearrow_forwardIf the following oligosaccharide was treated with an enzyme that cleaved only a 1,4 glycosidic bonds, what would be the products? OH CH₂OH OH CH₂OH OH CH₂OH OH OH OH OH CH₂OH CH₂OH a monosaccharide and a pentasaccharide a disaccharide and a tetrasaccharide two trisaccharides a monosaccharide, a disaccharide, and a trisaccharide O three disaccharides OH CH₂OH OH CH₂OH OH OH OH OHarrow_forward
- Could you help me with this? I'm stuck on this question.arrow_forwardh-t ownbid ㄴ HO. OH 시 3 애 애 HOW. X-glucose 근 6 HO Ito 5 4 open •애 3 애 1011. chain 애 5 How.ks 3 애 애 2 애기애 B-glucosearrow_forwardThe following chemical reaction is used to synthesize a flavouring agent that has an aroma similar to bananas. H₂SO4(aq) CH3COOH(1) + CH3(CH₂)₂OH(1) I || Identify the type of reaction that is represented by this synthesis. Select one: CH₂COO(CH₂)₂CH₂(1) + H₂O(1) IV O addition O hydrogenation O substitution O esterification O eliminationarrow_forward
- Select the glycoside(s) that are formed when the following monosaccharide is treated with CH3CH₂OH, HCI. HO OH OH HO- НО. OH OCH₂CH₂ OH OH OH OCH₂CH₂ OH OH OH -OH HO OH OH OH С HO- OH CH3CH₂OH HCI OH OH OCH₂CH3 -OCH₂CH3 a НО НО OH OH OH OH OH OCH₂CH₂ OCH₂CH₂ Lo -OH HO OCH CH НО. OH OH -0 OH OCH₂CH₂ OH -OH OCH₂CH₂arrow_forwardplease answer question 4arrow_forwardFollowing is a representation of the oxidation reaction for a monosaccharide. Identify the monosaccharide that is the reactant in this reaction. H HO Н HO НО. H НО H c=0 -Н OH -Н CH₂OH :0 OH -Н -ОН CH2OH ? [0] HO Н НО H OH -Н -ОН CH₂OHarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY