
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
- One mole of a monatomic ideal gas is held at the start at a pressure of 11 atm and 1 L. The gas undergoes isothermal expansion to 4 L followed by adiabatic expansion to 6 L. The gas is then isothermally compressed to 1.70 atm and adiabatically compressed back to 1 L.
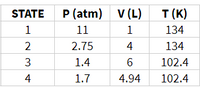
Transcribed Image Text:This table displays data for different states of a gas, with each state characterized by pressure (P), volume (V), and temperature (T).
| STATE | P (atm) | V (L) | T (K) |
|-------|---------|-------|-------|
| 1 | 11 | 1 | 134 |
| 2 | 2.75 | 4 | 134 |
| 3 | 1.4 | 6 | 102.4 |
| 4 | 1.7 | 4.94 | 102.4 |
### Description:
- **STATE**: Represents different conditions or phases of the gas.
- **P (atm)**: Pressure in atmospheres for each state.
- **V (L)**: Volume in liters for each state.
- **T (K)**: Temperature in Kelvin for each state.
This table can be used to explore the relationships between pressure, volume, and temperature in gas behavior, according to the principles of gas laws in thermodynamics.

Transcribed Image Text:The image displays a table intended for students to fill out as part of an educational activity related to thermodynamic cycles. The table includes several columns and rows to be completed as follows:
**Table Columns:**
1. **Process**: Lists the different processes within the cycle (1 ➡ 2, 2 ➡ 3, 3 ➡ 4, 4 ➡ 1).
2. **Q (kJ)**: Represents the heat transfer for each process in kilojoules.
3. **W (kJ)**: Stands for the work done in each process, also measured in kilojoules.
4. **ΔU (kJ)**: Refers to the change in internal energy for each process in kilojoules.
5. **ΔH (kJ)**: Indicates the change in enthalpy for each process in kilojoules.
6. **ΔS (J/K)**: Denotes the change in entropy for each process in joules per kelvin.
**Additional Task:**
- Part c asks to calculate the maximum efficiency for the cycle, based on the completed data in the table.
This table is part of an exercise to help students understand the relationships between heat transfer, work, and changes in thermodynamic properties like internal energy, enthalpy, and entropy.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 6 steps with 10 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemical-engineering and related others by exploring similar questions and additional content below.Similar questions
- Need a b carrow_forwardA diatomic ideal gas undergoes the exact same four step process as in problem 1A, starting at PointA (400 kPa, 600K, 1 L) A => B Isobaric expansion that triples the volume B => C Isochoric cooling to one-half the pressure C=> D Isobaric contraction to the original volume. D => A Isochoric heating to original pressure Determine the Pressure, Temperature and Volume at the end of each process. Determine the Change in the Internal Energy, Heat Flow and Work Done by the System during each process and for the entire cycle. Sketch a well labeled PV diagram for this cycle. Determine the Thermal Efficiency of this cycle (Work for Cycle/Heat Flow Into the System).arrow_forward5. If a gas of volume 6000 cm³ and at pressure of 100 kPa is compressed quasi-statically according to PV² = constant until the volume becomes 2000 cm³, determine the final pressure and the work transfer.arrow_forward
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The