
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
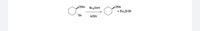
Transcribed Image Text:OMe
BuzSnH
OMe
+ BuzSnBr
"Br
AIBN
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Balance thechemical equation _CaCl2 (aq) + _Na2CO3 (aq) ------->_CaCO3 (s) + _NaCl (aq) Identify type ofreaction _NaCl (aq) + _AgNO3 (aq) -------->_NaNO3(aq) + _Agcl (s) Identify type ofreactionarrow_forwardMake sure answer has correct significant digitsarrow_forwardBalance CaCL2(aq) + Na3PO4(aq) —> Ca3(PO4)2(s)+ NaCL(aq). Identify single displacement /combination/ decomposition/double displacementsarrow_forward
- Balancing equations Fe + H2O Fe3 O4+H2arrow_forwardAaBbCcDdF 三 斗 T AaBbCcDdE AaBbCcAaBbCcDdE Aa v Normal Strong Emphasis Heading 1 v A v 5. What is the maximum amount of water that can be obtained from 22.05 g of copper and 750.00 mL of 1.00 M of nitric acid according to the below reaction? 3 Cu(s) + 8 HNO3(aq) 3 Cu(NO3)2(aq) + 2 NO(g) + 4 H20(t) 6. If 25.00 mL of 2.11x10-8 M Na2SO4 is mixed with 20.00 mL of 1.22x10-8 M Ba(NO3)2, how many grams of precipitate (which is?) can be formed? 7. In all the limiting reactant questions, how much of the excess reactant is left over? Give answer in mass or volume as appropriate. English United States O Focus MacBook Air 吕口 F3 000 FA F2 * ES > F6 F7 FB F9 F10 $4 & 4 6. 7 8 5 III III %#3 |II liliarrow_forwardComplete and balance gas-evolution equation. HC2H3O2(aq) + NaHSO3(aq) ------>arrow_forward
- proceed as W KNUD TE 6. Does the chemical reaction represented by 2Au(s) + 2HNO3(aq) →→→ 2AuNO3(aq) + H₂(g) proceed as written? Gold is a relatively useful metal for certain applications, such as jewelry and electronics. Does your answer suggest why this is so?arrow_forwardHd 10 5 0 5 mL of NaOH added 10 20 15 10 LO 5 0arrow_forward5) If all the NaHCO3 in bag 2 reacted, calculate the number of moles of gas produced? tates) E Focusarrow_forward
- pls answer all of them.arrow_forward100% Normal text Arial 11 B 1 - 1 2 3. 4 I5 6 7 8 T9 1 10 11 I 12 13 2. Copper (1) carbonate heptahydrate has the chemical formula a) CUCO3.5H20 b) CU2CO3.5H2O c) CuCoz. 7H20 d) Cu2CO3. 7H20 B. A double displacement reaction may be defined as: a) two or more simple substances producing a more complex substance *b) a more complex substance breaking down into simpler ones c) one element replacing another element in a compound d) a reaction involving two ionic compounds where the cations and anions replace each other Neutralisation occurs when: a) an acid is mixed with water b) a base is mixed with water c) a salt is mixed with an acid d) an acid is mixed with a base 山TOA Ai étv Book Proarrow_forwardALEKS - Alec Nema - Knowledge x C A Student Dissolves 12.2 G Of So x G Complete the table below, which x + A www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-lgNslkr7j8P3jH-IvUrTNdLZh5A8CnG03PBGuXr8iCPa7ZMmym9SYAHbJMKtSq7DrFR1fsYOuKny2zlevikulP55Fuy58W0qj2. E Apps Sprouts Academy. Online Tutoring 400 Request Heade. Q Weather & Soil CH. Ale Knowledge Check Question 29 Classify the substance shown in the sketch below. You can click the other tabs in the sketch to get a magnified view. Be sure you check all the boxes on the right-hand side that are correct for this substance. Note for advanced students: in some sketches the distance between particles has been exaggerated to make it easier to see each individual particle. classification substance (check all that apply) normal 1000X 10,000,000x Ogas Oliquid Osolid Delement Ocompound Omixture Osolution Opure substance Ohomogeneous mixture Oheterogeneous mixture I Don't Know Submit O 2020 McGraw-Hill Education. All Rights Reserved. Terms of Use |…arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY