
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:OH
H
OH
H
OH
H
H
OHN
HH JE
OH
HI
H
OH
F CH₂OH
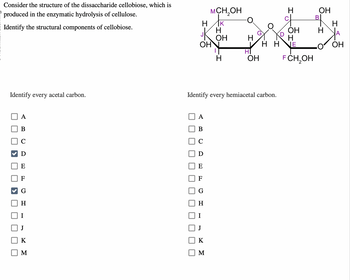
Consider the structure of the dissaccharide cellobiose, which is
produced in the enzymatic hydrolysis of cellulose.
Identify the structural components of cellobiose.
MCH₂OH
水
H K
H
Identify every acetal carbon.
A
B
0
D
E
L
Identify every hemiacetal carbon.
A
B
H
H
K
K
M
M

Transcribed Image Text:Identify every anomeric carbon.
B
E
F
H
K
M
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- esc ! 1 Take a look at this molecule, and then answer the questions in the table below it. CH₂OH OH Q H H OH H O Explanation H OH 2 H ▼ OH H O Is this a reducing sugar? CH₂ H OH H W O Does this molecule contain a glycosidic bond? H If you said this molecule does contain a glycosidic bond, write the symbol describing it. Check OH If you said this molecule does contain a glycosidic bond, write the common names (including anomer and enantiomer labels) of the molecules that would be released if that bond were hydrolyzed. If there's more than one molecule, separate each name with a comma. OH H # 3 E $ 4 R % 5 T A MacBook Pro 6 O yes O no O yes O no 0-0 - 0 V & 7 a В X * ローロ © 2023 McGraw Hill LLC. All Rights F 8 3arrow_forward12 CH2OH 1 H H 10 OH H H OH 9 6 H OH CH2 O. H H 4K OH OH 1 H H 12 OH isomaltose OH 3 a. Determine any acetal and hemiacetal in isomaltose. Carbon 7 is the acetal carbon. Carbon 1 is the hemiacetal carbon. b. Determine the numbering of each monosaccharide ring. The lower monosaccharide is numbered 5,4,3,2,1 v. The upper monosaccharide is numbered 7,8,9,10,11 c. Classify the glycosidic linkage as a or ß, and determine its location. The glycosidic linkage is B The linkage is 6 → 7. V d. Is the hemiacetal drawn as an a or ß anomer? The hemiacetal is a 00arrow_forwardName the types of glycosidc bonds for each structure below. You only need to name the type of bond.arrow_forward
- Using the structure below- classify digitoxins and digoxin as monosaccharides, oligosaccharides, or polysaccharides. Explain your classification?arrow_forwardHow many ketoses are shown in the figure below? C O C 10 OH ·OH a. 2 b. 0 c. 1 d. 4 e. 3 =C H-C- -OH HOTCH H OH HO -C-H HO CH HOTCIH H-C- -OH 卡 H H H 30arrow_forwardFollowing is a representation of the oxidation reaction for a monosaccharide. Identify the monosaccharide that is the reactant in this reaction. H HO Н HO НО. H НО H c=0 -Н OH -Н CH₂OH :0 OH -Н -ОН CH2OH ? [0] HO Н НО H OH -Н -ОН CH₂OHarrow_forward
- Take a look at this organic reaction: CH3- 0=0 C O NAD+ NADH CH₂_1-0 CH3—C−O + CO2 The reactant molecule is re-drawn in the drawing area below. Highlight in red any groups in this molecule in which carbon atoms are oxidized by the reaction. Highlight in blue any groups in which carbon atoms are reduced. If no carbon atoms are oxidized or reduced, check the box under the drawing area. _L_L- C CH₂ - No carbons are oxidized or reduced. Ćarrow_forwardCarbohydrates:arrow_forwardThe following two monosaccharides are what type of isomer? CHO CHO H- H- OH HO H. HO H. HO H- H OH OH CH,OH ČH,OH O a. anomers O b. ionomers O c. diastereomers O d. enantiomer O e. structural isomers Jump to.arrow_forward
- Is disaccharide “4” a reducing sugar?arrow_forwardHO HO ܦܐܐ ܒܐܠܥܐ HO HO OH OH HO- OH HO HO- OH Barley grain is used in the production of beer. This grain contains many polysaccharides which are hydrolyzed into sugars for yeast fermentation. Beta-glucans are polymers of glucose units. Many of these polysaccharides have a lot of branching. Identify the glycosidic linkages in this beta-glucan polysaccharide. OH OH OH HO Question 1.b of 45 HO OH A) a-1,4-linkages B) B-1,4-linkages C) a mix of a-1,3 and a-1,4-linkages D) a mix of B-1,3 and ß-1,4-linkages E) a mix of a-1,3 and ß-1,4-linkages Submit +arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY