
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
I am not understanding what is amphiprotic please example your answer thanks
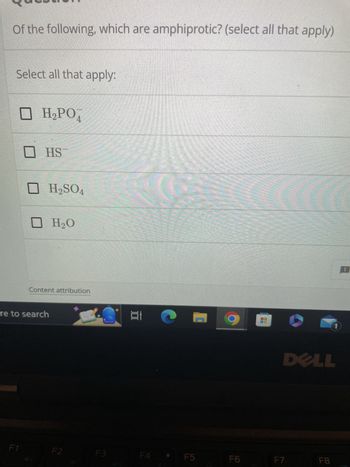
Transcribed Image Text:Of the following, which are amphiprotic? (select all that apply)
Select all that apply:
□ H₂PO4
F1
HS
re to search
H₂SO4
H₂O
Content attribution
F2
S Et
F3
F4
F5
A
a
F6
DELL
F7
1
F8
:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Page O Acids and Bases Calculating the pH of a dilute acid solution Calculate the pH of a 7.3 × 10 Maqueous solution of hydroiodic acid (HI). Round your answer to 2 decimal places. 0 Explanation 14 20 Check + S Main Menu 2023 McGarrow_forwardneed answerarrow_forwardHelp with the following question Round the answer to 2 decimal placesarrow_forward
- Give typed full explanation not a single word hand written otherwise leave itarrow_forwardHelp asaparrow_forwardQuestion 27 A. Given a solution with a hydroxide ion (OH") concentration of 4.30x108 M, what is the pH? B. Is the solution ACIDIC, BASIC or NEUTRAL? C. What is the pH of a solution that has a hydronium ion (H30*) concentration of 1.0x10-6 M? A Click Submit to complete this assessment. MacBook Air ********arrow_forward
- What would the pH be at the equivalence point when hydrochloric acid is neutralized with lithium hydroxide Less than 0 Less than 7 Approximately equal to 7 Greater than 7 Greater than 14arrow_forward17arrow_forwardK Suppose the pH of a solution decreases by 4. How does the hydrogen ion concentration change? H ew an example Get more help - (... The new hydrogen ion concentration is times the initial concentration. (Use scientific notation. Use the multiplication symbol in the math palette as needed.) Clear all Check answer Raining nowarrow_forward
- What is the hydrogen ion concentration of a solution with pH = 6.25? H*]= М about us contact us help privacy policy terms of use careers 12arrow_forwardTo name carboxylic acids use the name of the pare “_ Oi C 0ic " at the end. Example: Name the following: H. .C. C C. HO H. H. 188 エ エー○ エIarrow_forwardI need help with this practice test, this is not graded i just need help with it Please see attachedarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY