
Chemistry: The Molecular Science
5th Edition
ISBN: 9781285199047
Author: John W. Moore, Conrad L. Stanitski
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
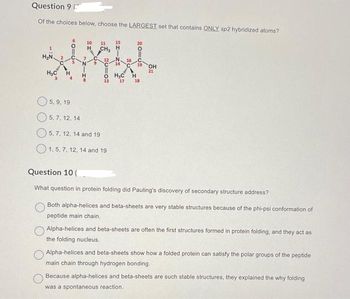
Transcribed Image Text:Question 9
Of the choices below, choose the LARGEST set that contains ONLY sp2 hybridized atoms?
H₂N 2
H₂C
5110
H
10 11
7
CH₂
12
15
H
5, 9, 19
5, 7, 12, 14
5, 7, 12, 14 and 19
1, 5, 7, 12, 14 and 19
O H₂C H
13 17
20=69
16
C 19
18
OH
21
Question 10 (
What question in protein folding did Pauling's discovery of secondary structure address?
Both alpha-helices and beta-sheets are very stable structures because of the phi-psi conformation of
peptide main chain.
O
Alpha-helices and beta-sheets are often the first structures formed in protein folding, and they act as
the folding nucleus.
Alpha-helices and beta-sheets show how a folded protein can satisfy the polar groups of the peptide
main chain through hydrogen bonding.
Because alpha-helices and beta-sheets are such stable structures, they explained the why folding
was a spontaneous reaction.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Cinnamaldehyde ocaus naturally in cinnamon oil. (a) What is the most polar bond in the molecule? (b) How many bonds and how many bonds are there? (c) Is cis-trans isomerism possible? If so, draw the isomers of the molecule. (d) Give the hybridization of the C atoms in the molecule. (e) What are the values of the bond angles 1, 2, and 3 ?arrow_forwardDetermine the hybridization state of each carbon atom in nemotin. HC ||| = C I с = ||| C T CH || C CH :0: CH :O: || с CH₂ CH₂arrow_forward6. Which of the following structure(s) is/are chiral? Br Br Br 1 Br 2 Br Br 3 Br Br 4arrow_forward
- HO: a :N CH3 -c=C-CH2 CH2 H3C Determine the hybridization and the approximate bond angles around the labeled atoms in this structure.arrow_forwardOxygenated hydrocarbons (compounds containing carbón, hydrogen, and oxygen) are common components of biofuels. The combustion of three isomers of C3H,0 (shown below) were studied to investigate the effects of structure on combustion speed and efficiency (Fuel 2010, 89, 2864-2872). Identify the number of sp3-hybridized carbon atoms in each of these isomers. Structure The number of sp3-hybridized carbon atoms (CH3)2CO HaCHOH CH3CH2CHOarrow_forwardCan you please check if the skeletal and Lewis structure are correct as well as the hybridization. Can you also explain the bonding interactions between each pair of adjacent C atoms?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning