
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
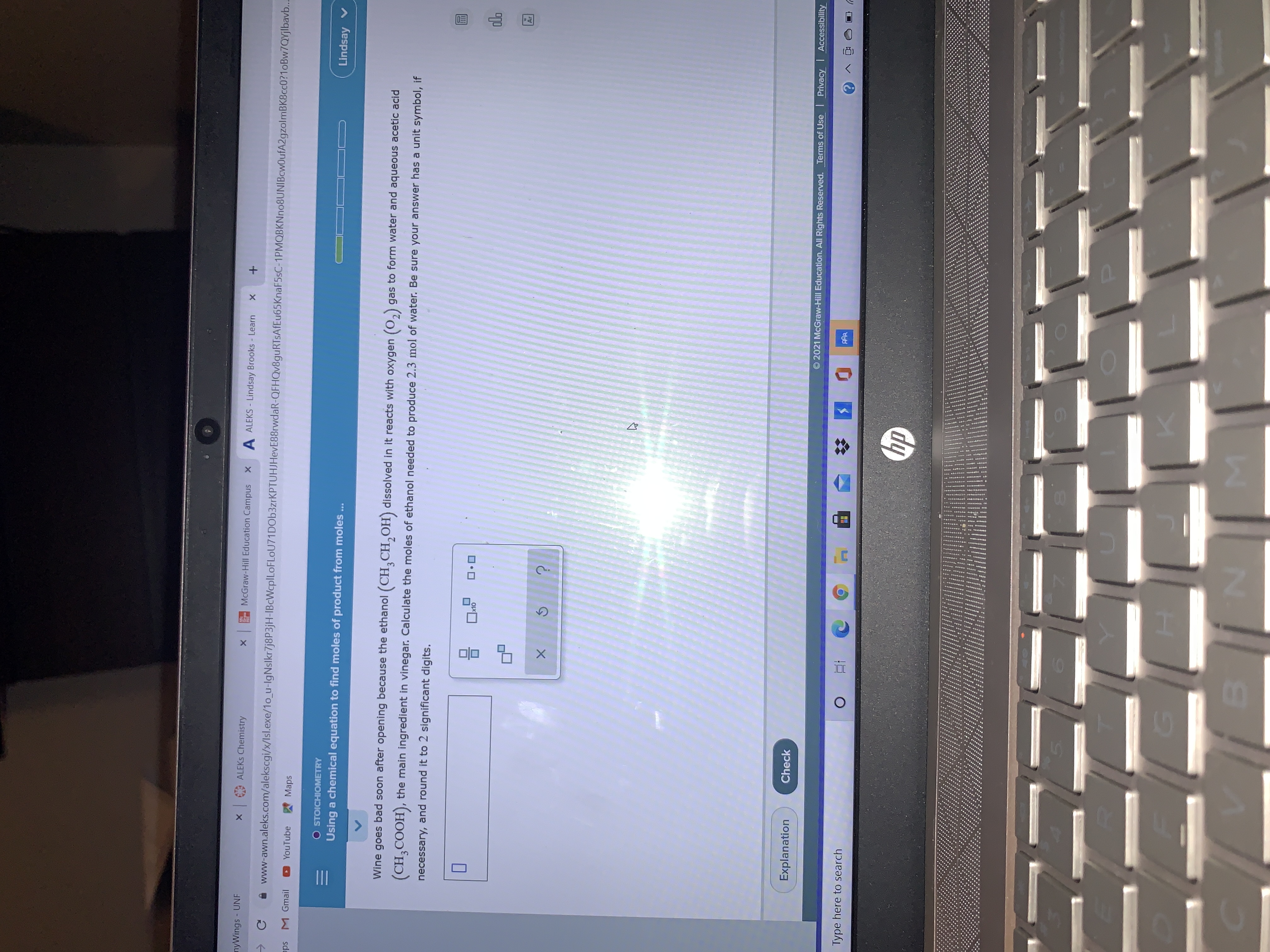
Transcribed Image Text:of product from moles...
Wine goes bad soon after opening because the ethanol (CH¸CH,OH) dissolved in it reacts with oxygen (0,) gas to form water and aqueous acetic acid
(CH,COOH), the main ingredient in vinegar. Calculate the moles of ethanol needed to produce 2.3 mol of water. Be sure your answer has a unit symbol, if
necessary, and round it to 2 significant digits.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Gaseous ammonia chemically reacts with oxygen (0,) gas to produce nitrogen monoxide gas and water vapor. Calculate the moles of oxygen needed to produce 2.5 mol of water. Be sure your answer has a unit symbol, if necessary, and round it to 2 significant digits. olo ?arrow_forwardplease make sure answer has the correct number of significant digitsarrow_forwardA student prepared benzil according to the experimental procedure using 2.58 g. of benzoin and 12 mL of nitric acid. The mass of the crude benzil product was 2.25 g. and the mass of the recrystallized benzil product was 1.95 g. Find the molecular weights for benzoin and benzil, then answer the following questions. What was the percent yield of the crude benzil? Assume units of %. Round answer to the first decimal place. What was the percent yield of the purified benzil? Assume units of % Round answer to the first decimal place. What was the percent recovery of the purified benzil from the crude benzil? Assume units of % Round answer to the first decimal place.arrow_forward
- Gaseous ethane (CH,CH,) reacts with gaseous oxygen gas (02) to produce gaseous carbon dioxide (CO,) and gaseous water (H,O). If 1.39 g of water is produced from the reaction of 1.5 g of ethane and 3.6 g of oxygen gas, calculate the percent yield of water. Be sure your answer has the correct number of significant digits in it. % ?arrow_forwardPart A. State a number of molecules for 1 mol of nitrogen, N2. Part B. State a mass for 1 mol of nitrogen, N2. Express your answer to two decimal places and include the appropriate units. Part C State the volume at STP for 1 mol of nitrogen, N2. Express your answer to three significant figures and include the appropriate unitsarrow_forward10 11 12 P13 Gaseous ammonia chemically reacts with oxygen (O,) gas to produce nitrogen monoxide gas and water vapor. Calculate the moles of ammonia needed to produce 1.30 mol of water. Be sure your answer has a unit symbol, if necessary, and round it to 3 significant digits. Submit Continuearrow_forward
- Ammonium phosphate (NH4), PO4) ("HN)) (H; PO,) with ammonia (NH, ). is an important ingredient in many fertilizers. It can be made by reacting phosphoric acid What mass of ammonium phosphate is produced by the reaction of 4.3 g of ammonia? Round your answer to 2 significant digits.arrow_forwardThis is the chemical formula for methyl tert-butyl ether (the clean-fuel gasoline additive MTBE): CH;OC(CH,), A chemical engineer has determined by measurements that there are 93.9 moles of hydrogen in a sample of methyl tert-butyl ether. How many moles of oxygen are in the sample? Be sure your answer has the correct number of significant digits. Omol ?arrow_forwardHow many moles of iron metal are required to react completely with with 406 mL of 1.59 mol/L copper(II) sulfate solution? Hint: Write a balanced chemical reaction equation first. Do not show your work in the space provided. Record only your final answer with the correct number of significant digits and the proper units.arrow_forward
- 12 Green plants use light from the Sun to drive photosynthesis, a chemical reaction in which liquid water and carbon dioxide gas form aqueous glucose (C6H₁2O6) and oxygen (0₂) gas. Calculate the moles of glucose produced by the reaction of 0.070 mol of carbon dioxide. Be sure your answer has unit symbol, if necessary, and round it to 2 significant digits.arrow_forwardGaseous methane (CH reacts with gaseous oxygen gas (0,) to produce gaseous carbon dioxide (CO,) and gaseous water (H,0). : If 1.01 g of carbon dioxide is produced from the reaction of 1.1 g of methane and 2.4 g of oxygen gas, calculate the percent yield of carbon dioxide. Be sure your answer has the correct number of significant digits in it.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY