
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
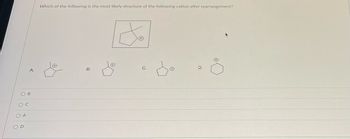
Transcribed Image Text:**Question:**
Which of the following is the most likely structure of the following cation after rearrangement?
**Diagram:**
The image displays a pentagon (cyclopentane ring) with a cation (+) symbol on a carbon atom connected to a methyl group, as the "following cation" that needs to be rearranged.
**Options:**
A. Cyclopentane ring with a cation (+) on a carbon next to where the methyl group was initially attached.
B. Cyclopentane ring with a cation (+) on the same carbon where the methyl group is attached.
C. Cyclopentane ring with a cation (+) on the carbon two positions away from where the methyl group is attached.
D. Cyclohexane ring with a cation (+) on one of the carbons (after ring expansion).
**Choices:**
- ○ B
- ○ C
- ○ A
- ○ D
---
This question references the concept of carbocation rearrangements, which is a topic commonly covered in organic chemistry. Carbocations may undergo rearrangement to achieve a more stable carbocation formation, such as moving from a less stable 1° to 2° or 3° carbocation or through ring expansions like 5 to 6 member cyclical compounds.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The stereochemistry of the following alkenes are? CH2 a. b. с. d. (A) a: Z; b: Z; c: E; d: E (D) a: Z; b: E; c: E; d: E (B) a: Z; b: Z; c: Z; d: E (E) a: Z; b: Z; c: E; d: Z (C) a: E; b: Z; c: E; d: Earrow_forwardShaw haw T compaunds YOIC a.Smm cauld prepare the fallauing materials with the starting shawn and ather any inarganie reagende necessarg arganic ar Jo HINT Yo ill peed EN do to allylic hreminatian anarrow_forward8:00 Problem 29 of 40 conc. HBr SOS 63 ..... Draw the products of the two step reaction sequence shown below. Use a dash or wedge bond to indicate stereochemistry of substituents on asymmetric centers, Ignore inorganic byproducts. H₂O Donearrow_forward
- Rank the following carbocations in order of increasing stability. H H. A D O A, B, D O D, A, B O B, A D O A, D, B Which of the following reactions would result in a 50/50 mixture of stereoisomeric products? HBr ? ..... REACTION A HBr ? REACTION B O Both reaction A and B O Neither reaction A nor B O Reaction B O Reaction A Ozonolysis of molecule A would result in the formation of what two functional groups? A O Two Aldehydes O An Aldehyde and a Carboxylic Acid O A Ketone and an Aldehyde O Two Ketonesarrow_forwardPlease provide IUPAC names:arrow_forwardOrder the following substrates in order of reactivity in substitution reactionsaromatic electrophilic (1 more reactive, 4 less reactive)arrow_forward
- What is the missing reagent in the reaction below? of of. cr CI Multiple Choice O LIAIH4. [2] H2O (1] CH2=CHLI, [2] H2O (1]) (CH2=CH)2CULİ, (2) H20 (1] DIBAL-H, (2] H20arrow_forwardNonearrow_forwardWhat is the final major organic product after these two consecutive steps? A. B. s ΟΑ ОВ SOC 1. HBr, ROOR 2. (CH3)2CuLi OD C. D. ?arrow_forward
- Use Zaitsev's Rule to predict the major product of the following E1 reaction? In töm Na Br XXXX Select one: a. I b. Il C. ||| d. IV IVarrow_forwardWhat reagents are needed to accomplish the following synthesis? A) H₂O/H* B CH3 E C) OH- H₂O/Peroxide ? BH 3 THF CH3 "OH 1. BH3 THF; 2. HO™, H₂O₂, H₂Oarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY