
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question

Transcribed Image Text:OA
B.
OC
D.
CE.
Compound
G is an Aldehyde
Compound H is a Carboxylic acid
Compound is an alcohol A
Compound B is a ketone
Compound is an Adehyde A
Compound B is a Ketone B
Compound B is an Aldehyde
Compound is a Ketone
Compound
is an Alcohol
Compound B is a Carboxylic acid
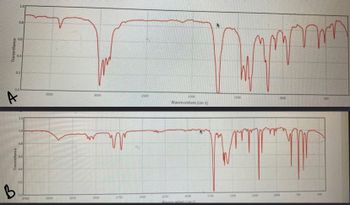
Transcribed Image Text:Transmitance
Transmitance
lot
90
B
90
0.2
0.0
0.4
3
0.5
04
20
0.0
3750
3500
COGE
OSCE
000€
DOO
ми
2750
0062
2500
2000
Wavenumbers (cm-1)
poor
1500
1700
السالم الله
1500
1250
www
DODE
0001
150
500
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Give the IUPAC name for the following compound. OH Select one: O A. 3-vinyl-1-butanol O B. 3-methyl-4-penten-1-ol O C. 3-methyl-1-pentenyl alcoholarrow_forward1. What functional group is produced when an aldehyde reacts with H2/Pt? A.secondary alcohol B. carboxylic acid C.hemiacetal D. primary alcohol E.alkane F.tertiary alcohol G. alkene 2. What reaction occurs when an aldehyde reacts with H2/Pt to form a primary alcohol? A. Hydration B. Hydration C. Dehydration D. Oxidation E. Reduction( hydrogentation) 3. What reaction occurs when an Ester react with H+/H2O to from a carboxylic acid and alcohol? A. Dehydration B. Reduction ( Hydrogenation) C.Hydrolysis D. Hydration E.oxidationarrow_forwardan acetal is a combination of what functional group a)an ester and an ether b)an ether and a hydroxyl , bonded to adjacent carbons c)two ethers d) an ether and a hydroxyl, bonded to the same carbonarrow_forward
- 1. H3C Type of functional group H3C Common Name 253 Understanding Organic Chemistry Workbook 3 Type of functional group m. Common Name O. CH3 Type of functional group n. CH3 Common Namearrow_forwardQUESTION 2 Classify the following as an Alcohol, Phenol, Thiol, Ether, Aldehyde or Ketone. CH2 CH2 H H3C OA Alcohol ОВ. phenol OC Thiol O D.Ether OE Aldehyde OF. Ketonearrow_forwardName each compound. A. B. C. CH₂CH3 CH₂CH3 a & CI CI CI Br. ď OH CI compound A name: compound B name: compound C name:arrow_forward
- Which of the following reactions can be used to make the following alcohol? OH 1. LIAIH4, ether 2. H30* 1. OsO4 2. NaHSO3, H20 1. CH3CH2MgBr, ether H. 2. H3O*, H2O 1. Hg(OAc)2, H2O/THF 2. NABH4 MacBook Proarrow_forward1. The functional groups present in the following molecules are: a. a carboxylic acid and an ester b. an ether, a ketone and a carboxylic acid c. an ester, a alcohol and a ketone d. an ether, a ketone and an alcohol c. nonc of the above H3C CH3 H3C- -CH2 CH2 .CH2 -CH2 HO, CH2 CH2arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY