
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:liamson Schools
9 Schoology
O My Profile - Zoom
b wcschools.schoology.com/common-assessment-delivery/start/4830003065?action%3Donresume&submissionld=4826983
E: Edulastic: Formativ.
D (26) we fell in love i.
6 ghj K 2020.11.12 Triangl.
Bookmarks
O(2) apocalypse - cig.
A Sso - Skyward
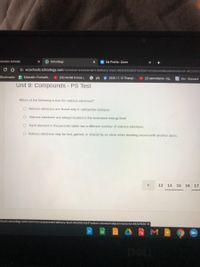
Unit 9: Compounds - PS Test
Which of the following is true for valence electrons?
O Valence electrons are found only in radioactive isotopes.
O Valence electrons are always located in the innermost energy level.
O Each element in the periodic table has a different number of valence electrons.
O Valence electrons may be lost, gained, or shared by an atom when bonding occurs with another atom.
13
14 15 16 17
chools.schoology.com/common-assessment-delivery/start/4830003065?action-onresume&submissionld=482698361#
DELL
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- The transition from theℓ = 2 to the ℓ = 1 state in CO is accompanied by the emission of a 9.55 x 10-4 eV photon. (a) Use this information to fi nd the rotational inertia of the CO molecule. (b) What is the bond length between the C and O atoms?arrow_forwardConsider the pure rotational spectrum of NO molecule. If the bond length of NO is 0.115 nm, calculate the wavelength corresponding to the transition with the rotational quantum number changing from J = 4 to J = 3.arrow_forwardWhat is the effect of temperature on breakdown voltage in a p-n junction?arrow_forward
- What are the popular methods of forming p-n junction?arrow_forwardThe electron affinity of Cl is 3.61 eV whereas the ionization energy of K is 4.34 eV. How far apart must two molecules be for the KC1 molecule to accumulate enough Coulomb energy to outpace the energy required to create the K+ and (I-) ions?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON