
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
For each of the following compounds, indicate the atom that is protonated when an acid is added to a solution of the compound.
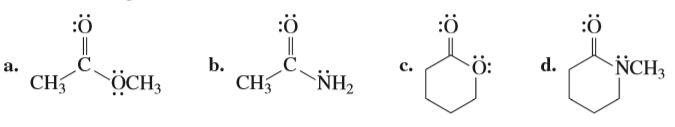
Transcribed Image Text::ӧ
:ӧ
:ö
||
:ӧ
||
NCH3
d.
Ӧ:
a.
b.
CH
ÖCH,
Nн,
CH3
ö:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- In a coordinate covalent bond: One of the shared electrons comes from one each of the two atoms in the bond One of the atoms in the bond provides both the electrons Neither of the atoms acts as an acid The electron sharing is the same as in a covalent bond Neither atom acts as an electron donorarrow_forwardTable 1: Concentrations of [H3O*] and [OH-] when various amounts hydrochloric acid (HCl, strong acid) or hypochlorous acid (HOC1, weak acid) are dissolved in water to make 1.00 L of solution at 25 Moles of acid added 0.00 0.30 mol HCI 0.75 mol HCI 1.00 mol HCI 0.30 mol HOCI 0.75 mol HOCI 1.00 mol HOCI - 1 - [H3O+] (M) 1.0 x 10-7 0.30 0.75 1.00 9.3 x 10-5 1.5 x 10-4 1.7 x 10-4 [OH-] (M) 1.0 x 10-7 3.3 x 10-¹4 1.3 x 10-14 1.0 x 10-¹4 1.1 x 10-10 6.8 x 10-11 5.9 x 10-11 1. For the strong acid, HCl, in model 1, why are there no HCl molecules present in the beaker? 2. For the weak acid in model 1, why are there mostly HA molecules present in the beaker? 3. From Table 1, what happens to [H3O*] as the number of moles of HOCI increases? 4. From Table 1, how are each of the entries for [OH-] related to the corresponding entries for [H3O+]? 5. If you hadn't been told that HOCI is a weak acid, how could you deduce that from Table 1?arrow_forwardWhich one of the following is characteristic of a base? O turns blue litmus red O produces HyO' in water O has a slippery, soapy fee O has a sour taste O is insoluble in water Question 2 Ammonia is a weak base because O it completely ionizes in aqueous solution it forms a dilute solution O it is only slightly soluble in water O it ionizes only slightly in water O it is a good acceptor of protonsarrow_forward
- 7. Note whether each substance is acid, basic, or neutral. baking soda baking powder vinegar window cleaner sprite lemon juice Immodium or milk of magnesia shampoo liquid soap bleacharrow_forwardWhich of the following anions will form a weakly basic solution? magnesium nitrate chloride bicarbonate ammoniumarrow_forwardStudents in a chemistry lab were using the 2 chemicals below in an experiment. Chemical pH Chemical #1 12 Chemical #2 Susan believes that Chemical #1 is a strong acid and that Chemical #2 is a strong base. Andrew believes that Chemical #1 is a strong base and that Chemical #2 is a strong acid. Which student is correct?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY