
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Draw resonance contributors for each of the following species and rank them in order of decreasing contribution to the resonance hybrid. Then draw the resonance hybrid.
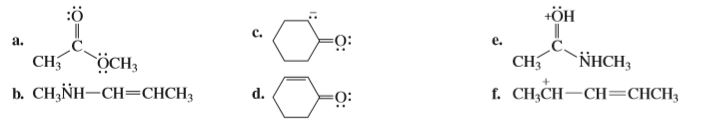
Transcribed Image Text::ö
+ÖH
a.
e.
CH
CH
NHCH,
ÖCH,
b. CH,NH-CH=CHCH3
f. CH;CH-CH=CHCH3
d.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Answer all the partsarrow_forwardEACTIVE Circle the better nucleophile in polar protic solvent. Below each answer, BRIEFLY explain your choice. a. Cl- and -I b. CH3SH and CH3OH DIVSO page 14 of 17arrow_forwardDraw the resonance contributors for the species/molecules shown in the boxes below. Then, indicate which species (if any) is the MAJOR contributor towards the resonance hybrid (most stable).arrow_forward
- c) Show Transcribed Text H₂SO4 Give a reasonable mechanism for this rxn, be sure to draw all intermidiates and use arrow pushing. Pay attention to syereochemistry and draw the most stable resonance contributer.arrow_forwardWhich of the following below would be considered a good Lewis Acid/Electrophile? A) OCH3 B) BF3 C) SCH3 D) CH3OH E) all of abovearrow_forwardAll resonance structures with arrow. Major resonance?arrow_forward
- 1. Consider two SN1 reactions: (A) water and tert-butylbromide and, (B) water and 2- bromopropane, both having the same concentration of reactants. a. Draw the reactants (nucleophile and substrate) for both reactions (A & B). b. Draw a curved arrow mechanism for the reaction between water and tert-butylbromide (A). Show all reactants, intermediates, and products do not show transition state structures. c. Draw products of each reaction (A & B). d. Predict which reaction will be faster (A or B). - e. Draw an energy vs. reaction coordinate diagram showing only the rate-determining step for both reactions on the same set of axes (axes available on the next page). Draw the reaction (A) with a dotted line and (B) with a solid line, and assume that the potential energies of the reactants are the same in both reactions (i.e. the only difference is in the energies of the two different transition states and resulting intermediates).arrow_forwardIn the picture, Each of the following compounds has more than one kind of (a-H's). First draw all a-H in, and circle the ones that are more acidic (that is, the ones more likely removed with a suitable base) in each compound. Thank you!arrow_forwardFor the reaction given below, draw a mechanism (curved arrows) and then predict which side of the reaction is favoured under equilibrium conditions. Include lone pairs and formal charges. Predict which side of the reaction is favored under equilibrium conditions, and explain your choice. a. the reactants are favored, because the negative charge on the electronegative oxygen atom is more stable (making it the stronger base). b. the products are favored, because the negative charge on the electronegative oxygen atom is more stable (making it the weaker base). c. the products are favored, because the negative charge on the large sulfur atom is more stable (making it the weaker base). d. the reactants are favored, because the negative charge on the large sulfur atom is more stable (making it the stronger base).arrow_forward
- Draw all resonance structure for each radical compound.arrow_forwardwe will discuss whether a methoxy group is electron donating or electron withdrawing. We will see that there is a competition between two factors. The methoxy group might be considered electron-withdrawing because of induction, or it might be considered electron-donating because of resonance. Below are the resonance structures of compound 1. Draw the missing curved arrow(s) to convert the each resonance structure into the next one...arrow_forwardDraw all the resonance contributors for the phenoxide in below. draw only one of the two equivalent "Kekule benzene" structures. include all valence lone pairs in answer.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY