
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
Transcribed Image Text:O Chemical Reactions
Using molarity to find solute moles and solution volume
1/5
Bish
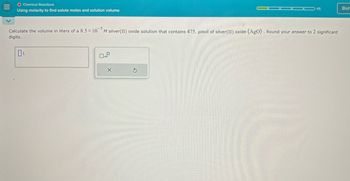
Calculate the volume in liters of a 8.5 x 105 M silver(II) oxide solution that contains 475. μmol of silver(II) oxide (AgO). Round your answer to 2 significant
digits.
OL
X
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Using molarity to find solute mass and solution volume Calculate the volume in liters of a 1.2 x 105 mol/L mercury(II) iodide solution that contains 75.0 g of mercury(II) iodide (Hg1₂). Round your answer to 2 significant digits. 0 OL X 3/5 3 ? dlaarrow_forwardCalculate the volume (in mL to 2 sig figs) of concentrated hydrochloric acid (12 M) needed to make 250.0 mL of a 1.5 M solution.arrow_forwardSuppose 9,07 g of potassium acetate is dissolved in 50. ml. of a 0.70 M aqueous solution of sodium chromate Calculate the final molarity of potassium cation in the solution. You can assume the volume of the solution doesn't change when the potassium acetate is dissolved in it. Round your answer to 2 significant digits.arrow_forward
- The concentration of acetylsalicylic acid in the original solution is 7.28 x 10-3 M. If the volume of this solution is 250.00 mL, calculate the moles of acetylsalicylic acid in this solution.arrow_forwardCalculate the mass in grams of NaF needed to prepare 0.50 L of 1.47 M NaF solution. Enter your answer with one decimal place. Do NOT include units.arrow_forwardSuppose 8.29 g of sodium bromide is dissolved in 200. ml. of a 0.50 M aqueous solution of potassium carbonate. Calculate the final molarity of bromide anion in the solution. You can assume the volume of the solution doesn't change when the sodium bromide is dissolved in it. Round your answer to 3 significant digits.arrow_forward
- A student needs to prepare 50.0 mL of a 1.20 M aqueous H2O2 solution. Calculate the volume of 4.6 M H2O2 stock solution that should be used to prepare the solution.arrow_forwardA solution of hydrochloric acid was formed by dissolving 5.51 g of hydrogen chloride gas in enough water to make 24.1 mL of solution. What is the concentration in mass/volume % of the solution?arrow_forwardWhat volume, in milliliters, of a 0.185 M solution of KCl contains 2.85 g of the compound? Express the volume to three significant figures and include the appropriate units. HA Value Units volume =arrow_forward
- Calculate the mass in grams of NaF needed to prepare 0.50 L of 2.25 M NaF solution. Enter your answer with one decimal place. Do NOT include unitsarrow_forwardA 9.33 % solution of sulfuric acid has a density of 1.07 g/mL. What is the molarity (M) of this solution? Enter only the numerical answer in the box . Give 3 sig figs in your answer.arrow_forwardWhat volume of 0.5175 M aluminum ion solution can be prepared from 6.0000 grams of aluminum sulfate? Show the numerical set-up(s), using correct units and the correct number of significant figuresarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY