
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Number 11-15. Identify if
Number 16-20. Draw the condensed structure
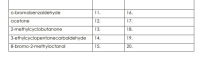
Transcribed Image Text:o-bromobenzaldehyde
11.
16.
acetone
12.
17.
2-methylcyclobutanone
13.
18.
3-ethylcyclopentanecarbaldehyde
14.
19.
8-bromo-2-methyloctanal
15.
20.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 1) The carbonyl group of aldehydes and ketones is polar where the ⸹+ symbol is placed on the oxygen atom and the ⸹- symbol is placed on the carbon atom. a) True b) False 2) Why is the boiling points of ethers similar to thise of alkanes instead of alcohols? a) Both ether molecules and alkanes molecules are polar, whereas alcohol molecules are nonpolar. b) Hydrogen bonding between ethr molecules is strong and this property is similar to that of alkanes. However, hydrogen bonding is weaker in alcohols than it is in both ethers and alkanes. c) Although ether molecules are polar, they cannot form hydrogen bonds with other ether molecules. Similarly, alkane molecules do not form hydrogen bonds, whereas there is hydrogen bonding in alcohols. d) None of these are correct explanationsarrow_forwardWhat functional groups are present in THC-O? Please circle on the chemical structure that is given.arrow_forwardDraw the structure for the given IUPAC naming (3S)-1-chloro-3-methyl hexanearrow_forward
- 1. Complete the following table: Name General Structure Example (condensed) Alcohols Ethers Aldehydes Ketones Carboxylic Acids Esterarrow_forwardNo Answer Question 25 of 25 Draw the condensed structure of 1,4-butanediol. 10 + 10 to enarrow_forwardWhen carbon bonds with other atoms to form a molecule, it must gain a share in how many electrons to complete its valence shell?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY