
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
Can you help me part G? Which is the formula of this rule of reaction?
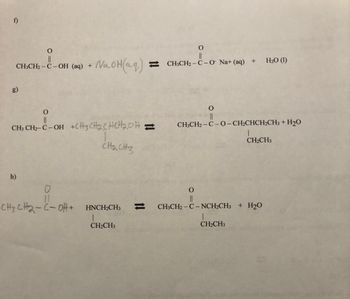
Transcribed Image Text:f)
O
CH3CH₂ - C - OH (aq) +
O
11
CH3 CH2-C-OH
h)
NaOH(aq)
+CH3CH₂CHCH₂OH;
CH₂ CH₂
0
CH3CH2-CoH+ HNCH2CH3
CH₂CH3
O
||
CH3CH2-C-O Na+ (aq) + H₂O (1)
O
CH3CH2-C-O-CH₂CHCH₂CH3 + H₂O
O
CH₂CH3
CH3CH2-C-NCH₂CH3 + H₂O
1
CH₂CH3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw the organic molecule(s) which is(are) formed in the following reaction. Do not include molecules like H₂O or HCl. (You have 2 chances until the answer will be given; you have already tried 0 times) H₂ CH CH₂ HC HC H C CH ***** CH Br + Compound #1 H₂C H₂ C. Draw only one molecule in each drawing box. NHarrow_forwardThe positive inductive effect (electron donor) of the alkyl groups stabilizes the positive charge of the carbocation. True or falsearrow_forwardThe starred O (on the reactant) is a heavy isotope of oxygen (18O). Predict the major product of this reactionarrow_forward
- omework 104 A CM/Team Time x Homework 103 x Hornework 102 structure.com/courses/943/assignments/16424 Homework 104 Due: Fri Mar 12, 2021 8:00am Unlimited Attempts Available 1. Given the following reaction, answer the questions that follow: C,H3 + 5 O2 → 3 CO, + 4 H,0 a) If you begin with 3.5 moles of C,H, and 6.2 moles of O,, find the moles of carbon dioxide produced. b) Find the mass of CO, produced using your work in step (a) above.arrow_forwardQ3. When the Organic Chemistry students carried out the experiment, they weighed out 5.651 g of p-aminophenol and mixed it with excess acetic anhydride. (Show ALL steps and mathematical equations involved in your calculations to help the students. Remember to label all steps clearly and use appropriate units in each step). Your job is to serve as their tutor so you need to be as clear as possible! e.g. Step 1: Use equation ….., Step 2: multiply by …. a. Calculate the moles of p-aminophenol (report your answer to the correct number of significant figures) b. Calculate the theoretical yield of Acetaminophen (Paracetamol) in moles (report your answer to the correct number of significant figures) c. Calculate the theoretical yield of Acetaminophen (Paracetamol) in grams (report your answer to the correct number of significant figures)arrow_forwardhelp please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all workingarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY