
Introductory Chemistry: A Foundation
9th Edition
ISBN: 9781337399425
Author: Steven S. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:76°F
Sunny
FI
1
7
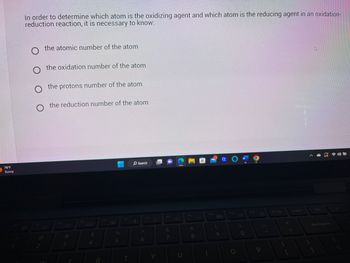
In order to determine which atom is the oxidizing agent and which atom is the reducing agent in an oxidation-
reduction reaction, it is necessary to know:
O
O the oxidation number of the atom
O the protons number of the atom
the reduction number of the atom
O
the atomic number of the atom
F3
FBI
3
F2
2
$
4
R
F5
ME
%
5
F6
T
Search
F7
A
6
F8
&
7
U
F9
*
8
Sp
F10
(
9
a
F11
O
0
F12
P
Prt Sc
{
+
Insert
=
Del
Backspace
40 t
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- . For each of the following unbalanced oxidation-reduction chemical equations, balance the equation by inspection, and identify which species is the reducing agent. a.Fe(s)+O2(g)Fe2O3(s)b.Al(s)+Cl2(g)AlCl3(s)c.Mg(s)+P4(s)Mg3P2(s)arrow_forwardThe iron content of hemoglobin is determined by destroying the hemoglobin molecule and producing small water-soluble ions and molecules. The iron in the aqueous solution is reduced to iron(II) ion and then titrated against potassium permanganate. In the titration, iron(ll) is oxidized to iron(III) and permanganate is reduced to manganese(II) ion. A 5.00-g sample of hemoglobin requires 32.3 mL of a 0.002100 M solution of potassium permanganate. The reaction with permanganate ion is MnO4(aq)+8H+(aq)+5Fe2+(aq)Mn2+(aq)+5Fe3+(aq)+4H2O What is the mass percent of iron in hemoglobin?arrow_forwardFor each of the following balanced half-reactions, determine whether an oxidation or reduction is occurring. (a) Cl+3eCl2 (b) Mn2+MnO2 (c) H2H+ (d) NO3NOarrow_forward
- A solution contains both iron(II) and iron(III) ions. A sample Of the solution is titrated with 35.0 ml, of M KMnO4, which oxidizes Fe2+ to Fe3+. The permanganate ion is reduced to manganese(ll) ion. The equation for this reaction is MnO4(aq)+8 H+(aq)+5Fe2+(aq)Mn2+(aq)+5Fe3+ +4H2OAnother 50.00-mL sample of the solution is treated with zinc, which reduces all the Fe3+ to Fe2+. The equation for this reaction is 2Fe3+(aq)+Zn(s)2Fe2+(aq)+Zn2+(aq)The resulting solution is again titrated with 0.0280 M KMnO4; this time 48.0 ml, is required. What are the concentrations of Fe2+ and Fe3+ in the solution?arrow_forwardSpecify which of the following equations represent oxidation reduction reactions, and indicate the oxidizing agent, the reducing agent, the species being oxidized, and the species being reduced. a. CH4(g)+H2O(g)CO(g)+3H2(g) b. 2AgNO3(aq)+Cu(s)Cu(NO3)2(aq)+2Ag(s) c. Zn(s)+2HCl(aq)ZnCl2(aq)+H2(g) d. 2H+(aq)+2CrO42-(aq)Cr2O72-(aq)+H2O(l)arrow_forwardComplete and balance each of the following half-reactions (steps 2—5 in half-reaction method): (a) Sn4+(aq)Sn2+(aq) (b) [Ag( NH 3)2]+(aq)Ag(s)+NH3(aq) (c) Hg2Cl2(s)Hg(l)+Cl(aq) (d) H2O(l)O2(g) (in acidic solution) (e) IO3(aq)I2(s) (f) SO32(aq)SO42(aq) (in acidic solution) (g) MnO4(aq)Mn2+(aq) (in acidic solution) (h) CI(aq)CIO3(aq) (in basic solution)arrow_forward
- Consider the following balanced redox reaction in basic medium. 3Sn2+(aq)+Cr2O72(aq)+4H2O3Sn4+(aq)+Cr2O3(s)+8OH(aq) (a) What is the oxidizing agent? (b) What species has the element that increases its oxidation number? (c) What species contains the element with the highest oxidation number? (d) If the reaction were to take place in acidic medium, what species would not be included in the reaction?arrow_forwardSuppose you have three different metals. A, B, and C. When metals A and B come into contact. B confides and A does not corrode. When metals A and C come into contact, A corrodes and C does not corrode. Based on this information, which metal corrodes and which metal does not corrode when B and C come into contact?arrow_forwardComplete and balance the following oxidation-reduction reactions, which give the highest possible oxidation state for the oxidized atoms. (a) Al(s)+F2(g) (b) Al(s)+CuBr2(aq) (single displacement) (c) P4(s)+O2(g) (d) Ca(s)+H2O(l) (products are a strong base and a diatomic gas)arrow_forward
- One of the few industrial-scale processes that produce organic compounds electrochemically is used by the Monsanto Company to produce1,4-dicyanobutane. The reduction reaction is 2CH2CHCH+2H++2eNC(CH2)4CN The NC(CH2)4CN is then chemically reduced using hydrogen gas to H2N(CH2)6NH2, which is used in the production of nylon. What current must be used to produce 150.kg NC(CH2)4CN per hour?arrow_forwardAn active (metal) electrode was found to gain mass as the oxidation-reduction reaction was allowed to proceed. Was the electrode part of the anode or cathode? Explain.arrow_forwardIdentify the following as an oxidation, a reduction, a decomposition, or a dismutation reaction: Cl2+2e2Cl a.a reduction b.an oxidation c.a decomposition d.a dismutationarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning