
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
nurse measures the volume of a drop of medicine. The volume of 1 drop of medicine is 0.87mL. What is the volume in deciliters of 1000drops of medicine? Write your answer as a decimal.
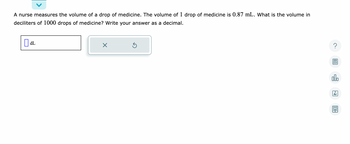
Transcribed Image Text:A nurse measures the volume of a drop of medicine. The volume of 1 drop of medicine is 0.87 mL. What is the volume in
deciliters of 1000 drops of medicine? Write your answer as a decimal.
dL
X
Ś
?
olo
Ar
191
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- You have a stock NaCI of 5 g/L. You want to prepare 259 mL of a 1 mg/L NaCI solution. How many mL of the stock solution will you need? Give your answer with two digits after the decimal.arrow_forwardA tortoise ambles along at 64.6 mm/s. What is this in miles/hr ? Use 1 = 1.609 km in your calculations.arrow_forwardA chemist makes 730.mL of potassium dichromate K2Cr2O7 working solution by adding distilled water to 220.mL of a 0.139molL stock solution of potassium dichromate in water. Calculate the concentration of the chemist's working solution. Round your answer to 3 significant digits.arrow_forward
- The following densities resulted from a student's measurements. What is the mean of their densities? Include units in your answer. Density 1 = 8.51 g/mL Density 2 = 9.97 g/mL Density 3 = 9.07 g/mLarrow_forwardGive Detailed Solution (don't give Handwritten answer)arrow_forwardA chemist adds 175.0 mL of a 28.8 g/dL calcium bromide (CaBr2) solution to a flask. Calculate the mass in grams of calcium bromide the chemist has added to the flask. Round your answer to 3 significant digitsarrow_forward
- A student was given an unknown metal. The student determined that the mass of the metal was 30.2 g. The student placed the metal in a graduated cylinder filled with 20.0 mL of water. The metal increased the volume of water to 22.9 mL. Calculate the density of the metal and determine the identity of the metal using the table below.arrow_forwardSuppose you are trying to find the volume of a box based on the given measurements for the length, width, and height of the box, where the height was measured in two parts. length, l = 2.20 in width, w = 3.71 in height, h = 10.4 in + 1 in Calculate the height, ℎ, of the box, first keeping all digits, then rounding to the proper number of significant figures. unrounded h = ____in rounded h =____ in Calculate the volume (V) of the box using V =l x w x h. Round to the proper number of significant figures. v=_____in3arrow_forwardMultiply or divide the following measurements. Be sure each answer you enter contains the correct number of significant digits. 20.94 cm x 80 cm - 93. g ml. -* 19. mL - 946.2 mol 31.5 L - 0 cm 2 mol 0.9 Xarrow_forward
- A chemist weighed out 7.2g of mercury. Calculate the number of moles of mercury she weighed out. Be sure your answer has the correct number of significant digits.arrow_forwardA surveyor measures the length of a surveying chain containing 1000 chain links. The length is 0.0489 km. What is the length of a single chain link in centimeters? Write your answer as a decimal. cm Xarrow_forwardA chemistry student needs 35.0 mL of dimethyl sulfoxide for an experiment. By consulting the CRC Handbook of Chemistry and Physics, the student discovers that the density of dimethyl sulfoxide is 1.10 g·cm -3 Calculate the mass of dimethyl sulfoxide the student should weigh out. Be sure your answer has the correct number of significant digits. ?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY