
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
i understand this have to be hydrolized, please explain what im doing wrong. do you have any tips in how i can break the problem up ?
Part A was marked wrong
Part B, I kinda dont know to break it apart....
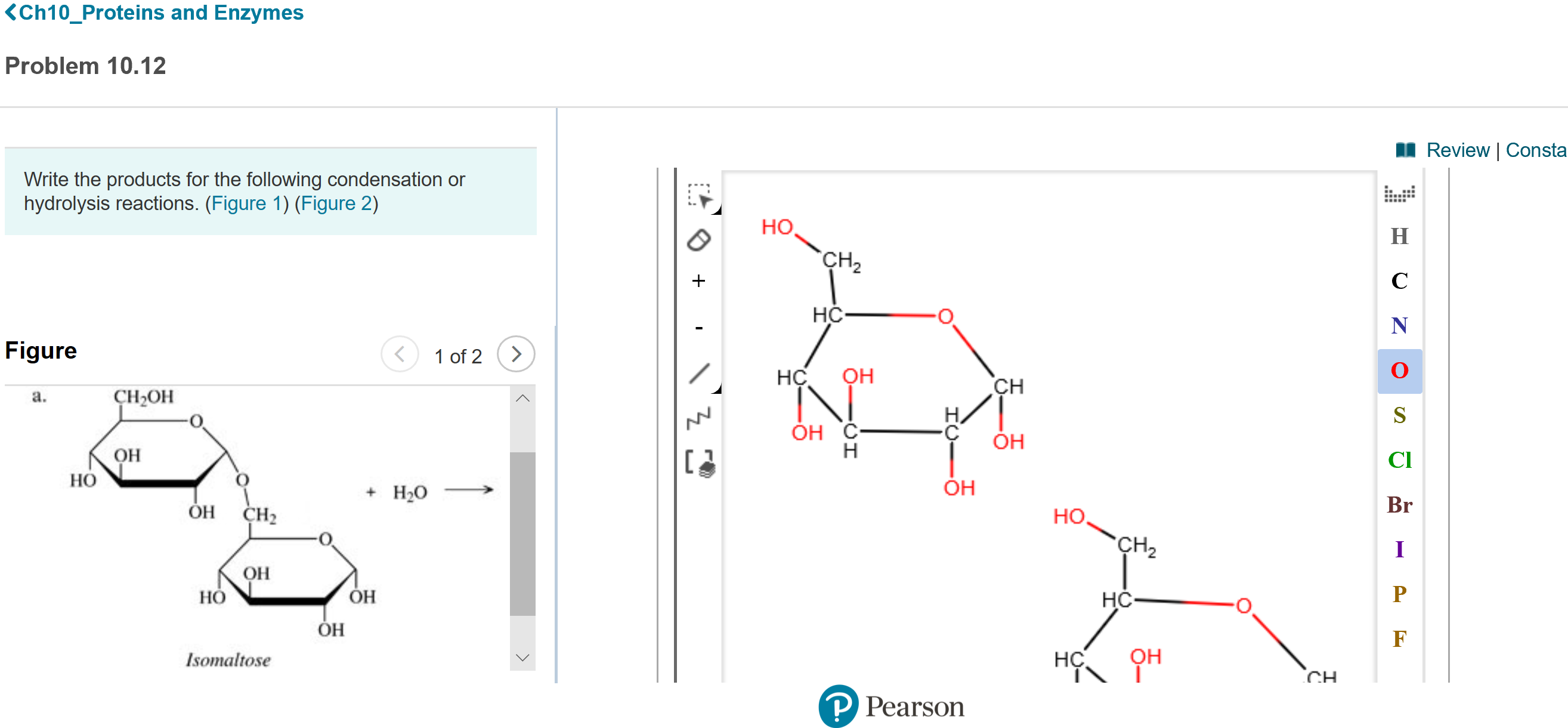
Transcribed Image Text:<Ch10_Proteins and Enzymes
Problem 10.12
Review | Consta
Write the products for the following condensation or
hydrolysis reactions. (Figure 1) (Figure 2)
но,
Н
сна
нс-
Figure
1 of 2
<>
НС.
он
CH
a.
CH-ОН
Он С-
н
Он
Он
CI
НО
+ H2O
Он
ОН
ҫH
но,
Br
"Cна
Он
Но
ОН
НС-
P
Он
Isomaltose
Он
CH.
P Pearson
エ
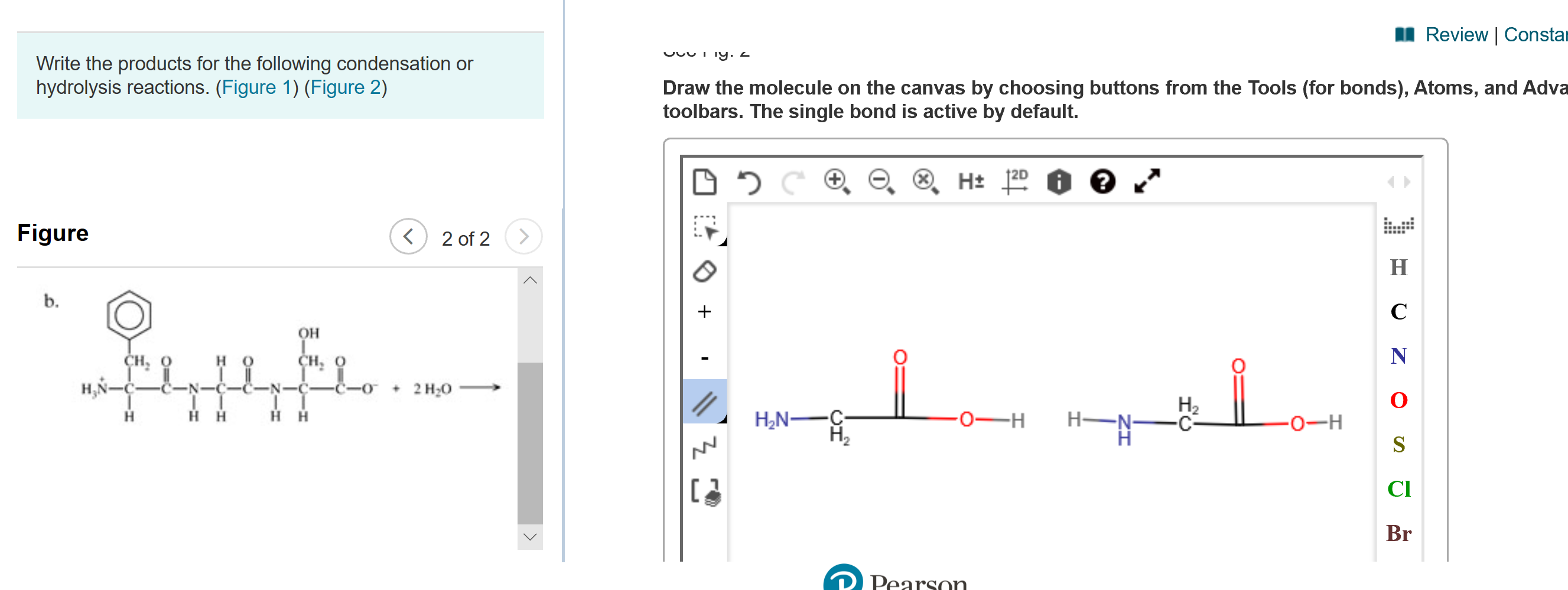
Transcribed Image Text:I Review | Constar
Write the products for the following condensation or
hydrolysis reactions. (Figure 1) (Figure 2)
Draw the molecule on the canvas by choosing buttons from the Tools (for bonds), Atoms, and Adva
toolbars. The single bond is active by default.
Figure
く
2 of 2
н
b.
Он
сн, о
ҫн, о
+ 2 H;0
-C-
H-N-
На
нн
H,N-
-0-H
[
CI
Br
Pearson
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- PROCEDURE:A. TESTS FOR METHYL ALCOHOL 1. Salicylic Acid Test. Methyl alcohol with salicylic acid and concentrated sulfuric acid willgive methyl salicylate or oil of wintergreen, a compound with an odor of peppermint.In a test tube containing 0.2 gm of salicylic acid, add 1 ml. of concentrated sulfuric acid(CAUTION!). Introduce 1 ml. of methanol into the test tube. Result:arrow_forwardNow that you know the moles of NaOH used for each trial as well as the volume of NaOH used for each trial, calculate the concentration of the NaOH that you made in lab for each trial. Round to the ten-thousandths place please.arrow_forward= Applying like dissolves like For each solute, click the button under the better solvent. : 0: || с HO- solute NH₂Br : 0: || CH₂CH₂C- OH CH, (CH₂) CH₂ Which is the better solvent? H H CH₂ CH₂OH CH₂ O : 0: CH₂-S - CH₂ CH₂CH₂OH O H₂O CC14 H H 1/5arrow_forward
- Question: Identify products A and B giving evidence from the student's observations and the data provided. *ive sent a picturearrow_forwardMultiple Choice Each of the numbered items or incomplete statements is followed by answers or by completions of the statement. Select the one lettered answer or completion that is best in each case. Write your answer before each number. 48.A substance that has a high degree of purity and is used in direct standardization purposes: a. Analyzed reagent C. USP grade reagent b. Primary standard reagent d. Technical grade reagent 49. When a weak acid is titrated with strong base, the indicator used is a. Methyl red C. Titration not possible Methyl orange b. phenolphthalein d. 50.An organic compound that changes from one color to another at a certain pH is called: a. Indicator C. Standard solution b. Buffer d. Test solutionarrow_forwardHalf of the solution is cut off, can you please re-write more vertically? Thank youarrow_forward
- 0-H Oxone/ NaBr. 13.arrow_forward40 mg of sodium hydroxide is required to saponify 1gm sample, this means that (MW of NaOH= 40 g/mole, MW of KOH is 56g/mol) O a. saponification value equals 56 O b. acid value equals 40 O C. Acid value equals 56 O d. saponification value equals 40arrow_forwardSelect one for each boxarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY