
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
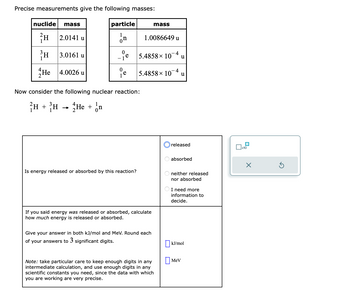
Transcribed Image Text:Precise measurements give the following masses:
nuclide mass
²H
2.0141 u
il
He 4.0026 u
3.0161 u
particle
In
- ie
0
e
Now consider the following nuclear reaction:
H + H → He + n
Is energy released or absorbed by this reaction?
1.0086649 u
4
5.4858 × 10 u
5.4858× 10-4 u
mass
If you said energy was released or absorbed, calculate
how much energy is released or absorbed.
Give your answer in both kJ/mol and MeV. Round each
of your answers to 3 significant digits.
Note: take particular care to keep enough digits in any
intermediate calculation, and use enough digits in any
scientific constants you need, since the data with which
you are working are very precise.
released
absorbed
neither released
nor absorbed
I need more
information to
decide.
kJ/mol
Mev
x10
X
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- When 55Fe undergoes electron capture, what is the immediate nuclear product? 54Fe 55Mn 55Fe 55Coarrow_forwardSuppose a yttrium-88 nuclide transforms into a strontium-88 nuclide by absorbing an electron and emitting a gamma ray. Complete the nuclear chemical equation below so that it describes this nuclear reaction. 88 0 39 Y +_ je → D X Y Sarrow_forwardA moon rock collected by a U.S. Apollo mission is estimated to be 4.00 billion years old by uranium/lead dating. Assuming that the rock did not contain any lead when it was formed, what is the current mass of Pb206 in the rock, if it currently contains 1.430 g of U238? The half-life of U238 is 4.47×109 years.arrow_forward
- Calculate the energy released (in J) from the following nuclear reaction: 국H + 3H→He + 할 H = = 1.007 825 032 24 amu H = 2.014 101 778 11 amu H = 3.016 049 281 99 amuarrow_forwardIdentify the unknown particle X in the following nuclear reaction equations. (Enter your answers in the form A X Z 10n + 2713Al → X + 42He 11H + 189F → 158O + X 42He + X → 2512Mg + 10n 10n + 23592U → 13855Cs + X + 2 10n Identify the unknown particle X in the following equation for fission. (Enter your answer in the form A X Z .) 23692U → X + 9436Kr + 3 10narrow_forwardRuthenium-106 (106Ru) is used to treat melanoma in the eye. This isotope decays by β– emission with a half-life of 373.59 days. One source of the isotope is reprocessed nuclear reactor fuel. How many protons and neutrons does the 106Ru nucleus contain? Identify the missing daughter nucleus in the β– emission decay of 106Ru below. How many years after 106Ru is implanted in the eye does it take for its decay rate (or activity) to be reduced to 27.5% of the initial decay rate? Use 1 year = 365 days.arrow_forward
- Balance the following nuclear reaction: 4He2 + 14N7 → 17O8 + ?arrow_forwardThe only stable isotope of iodine is iodine-127. Predict the mode of decay of iodine- 125. Write the corresponding nuclear reaction that occurs as the radioactive isotope, iodine-125 decays to a more stable form.arrow_forwardBalance the following nuclear reactionsarrow_forward
- 1. Complete the following nuclear equations. Indicate the symbol, the mass number, and the atomic number of the unknown particle. ? 181 131 (a) 331 → ß + ? (b) 74 W + °e → ? → '"N + ? (d) Sr → ? 90 (c) 0 → + -iß; 38 (e) ? 226 Ra + He; 239 Pu → ? +He; Не; 88 94 (g) U + ¿n –→ " 90 Sr + ? _ + 3,n; (h) 2Cf → 193zr + ? + 4;n 92 38arrow_forwardX * Cengage Digital Learning Fill in the missing symbol in X www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-Ivd38R-w7xPuAcyA O NUCLEAR CHEMISTRY Balancing a nuclear chemical equation Fill in the missing symbol in this nuclear chemical equation. 1. H' + 0 H + o - ƏH + 0 2. 8. Check Explanationarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY