
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
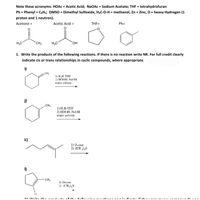
Transcribed Image Text:**Educational Resource: Understanding Organic Chemistry Reactions**
### Important Acronyms:
- **HOAc**: Acetic Acid
- **NaOAc**: Sodium Acetate
- **THF**: Tetrahydrofuran
- **Ph**: Phenyl = C₆H₅
- **DMSO**: Dimethyl Sulfoxide
- **H₃C-O-H**: Methanol
- **Zn**: Zinc
- **D**: Heavy Hydrogen (1 proton and 1 neutron)
### Chemical Structures:
1. **Acetone**:
- Structure: \( \text{H}_3\text{C-CO-CH}_3 \)
2. **Acetic Acid**:
- Structure: \( \text{H}_3\text{C-COOH} \)
3. **THF (Tetrahydrofuran)**:
- Structure: A cyclic ether with the formula \( \text{C}_4\text{H}_8\text{O} \).
4. **Ph (Phenyl)**:
- Structure: The phenyl group is represented as a benzene ring, \( \text{C}_6\text{H}_5 \).
### Reaction Exercise:
**1. Write the products of the following reactions. If there is no reaction, write NR. For full credit, clearly indicate cis or trans relationships in cyclic compounds, where appropriate.**
#### Reactions:
- **i)**
- Reactant: Cyclohexene compound with a methyl group
- Reagents:
1) \( \text{H}_3\text{B}\), THF
2) \( \text{HOOH, NaOH} \), water solvent
- **j)**
- Reactant: Cyclohexene compound with a methyl group on a different position
- Reagents:
1) \( \text{H}_3\text{B}\), THF
2) \( \text{HOOH, NaOH} \), water solvent
- **k)**
- Reactant: A linear alkene with a methyl group
- Reagents:
1) Ozone
2) \( (\text{CH}_3)_2\text{S} \)
-
Expert Solution
arrow_forward
Step 1
Given:
- Hydroboration - oxidation reaction
- Ozonolysis
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Each row represents an experimental “run” (in this case, averaged sample of mango wine). Column A shows the run number; Column B shows the percentage of ethanol; Column C shows the amount of glycerol (in g/L); Column D shows the amount of acid (in g/L); Column E shows the temperature of the run (in °C); and Column F shows the pH of the run. Y represents Ethanol of mango wine. 1. Create a scatterplot of the ethanol data for all 20 runs a. Draw a graphical representation of the model that you chose to use. b. Comparing the model to your data, evaluate its fit in one or two sentences. (Not sure what this question is getting at. I've provided all the information given) c. Replace the generic Yi = β0 + ε with the information you have here. Please help. I have no idea what this even means or where to start. HERE is the attached dataset to start with; a b c d e f Run Ethanol Glycerol Acidity Temp pH 1 4.8 3.5 0.84 24 3.8 2 9.6…arrow_forwardWhat is the pH of a solution when [-OH] = 10-12 M? Group of answer choices pH = 0 pH = 2 pH = 10 pH = 12 pH = 14arrow_forwardConsider the following data on some weak acids and weak bases: acid base K. K, name formula name formula HCH;CO, 1.8 × 10 5 -4 acetic acid ethylamine C,H;NH, 6.4 x 10 C,H;NH, 4.3 × 10 - 10 hydrocyanic acid HCN 4.9 × 10 - 10 aniline Use this data to rank the following solutions in order of increasing pH. In other words, select a 'l' next to the solution that will have the lowest pH, a '2' next to the solution that will have the next lowest pH, and so on. solution pH 0.1 M C2H5NH3Br choose one 0.1 M C6H5NH3CI choose one v 0.1 M NaCN choose one v 0.1 М KСI choose one varrow_forward
- The acid dissociation constant K of trimethylacetic acid (HC (CH3)3 CO₂) CO2) is 9.33 × 106. Calculate the pH of a 0.93M solution of trimethylacetic acid. Round your answer to 1 decimal place. pH ☐arrow_forwardHenderson-Hasselbalch equation for a propanoic acid solution (CH3CH2CO2H, p?a=4.874). Using the equation to calculate the quotient [A−]/[HA] at three different pH values. pH=4.311 pH=4.874 pH=5.061arrow_forwardCalculate the pH of a 0.591 M aqueous solution of benzoic acid (C6H₂COOH, K₂ = 6.3x10-5). pH =arrow_forward
- CH;COOOH 3. 3/9 Ph Ph Ph 4. ... ...arrow_forwardAnswer to question 20 pleasearrow_forwardThe acid dissociation constant K of trimethylacetic acid (HC(CH³)₂CO₂) is 9.33 × 10¯6. a Calculate the pH of a 3.6M solution of trimethylacetic acid. Round your answer to 1 decimal place. pH = - 0 X Śarrow_forward
- The structure of benzene-1,2,3-tricarboxylic acid (pKa1 =2.88, pKa2 4.76, pKa3 = 7.13) can be seen on the right. What is the predominate form of benzene-1,2,3-tricarboxylic acid in a solution that has a pH of 6.0?arrow_forwardCalculate the pH and fraction of dissociation (?) for each of the acetic acid (CH3COOH, p?a=4.756) solutions. A 2.15*10^-12 M solution of CH3COOHCH3COOH.arrow_forwardIf the [OH-] = 1 x 10-4M, which is true? C pH = 4 and [H3O+] = 1 × 10-4M pH = 4 and [H3O+] = 1 x 10-10M O pH = 10 and [H3O+] = 1 x 10-4M pH = 10 and [H3O+] = 1 x 10-10Marrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY