
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Kk.266.
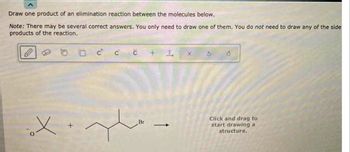
Transcribed Image Text:Draw one product of an elimination reaction between the molecules below.
Note: There may be several correct answers. You only need to draw one of them. You do not need to draw any of the side
products of the reaction.
C C
с +
X.gh.
Br
1
I
x
Click and drag to
start drawing a
structure.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 28. (a) In Figure P7.7, which sphere could represent a Na atom, Nation, and K atom? Provide an explanation for your choice. (b) In Figure P7.7, which sphere could represent an O atom, S atom, and S2- ion? Provide an explanation for your choice. FIGURE P7.7 (b) (c)arrow_forward3) In a far away land and long ago, a series of experiments were carried out. Three samples of minerals where collected, and the sample were analyzed, and found to contain two elements kalium and pyron. . They use K for kalium and HO for pyron. Sample..... A (from a mine). . B (from a dry lake).. . .. C (from the foundation of a castle). 0.8729 Mass (g) g of Kalium g of Pyron 0.5236 0.2755 0.2481 0.6918 0.4770 0.2148 0.4592 0.4137 Use the Law of Conservation of Mass to determine if the data is worth using. sample A (froma mine) (g) 0. S236 sample B Cfrom O.6918 (9) o. 2755 + O 248TED Thus every sample has different vawes of the Same element, the mess of each sample is equal to the addifio of itscomponents, wich states that the mess of the procloct matches the mass of the' sample. dry lake) 9) a 0.4770 + 0.2148 Io sample c (frome fondatior ofecosfle O, 8729 0.4592 K+ 0.4137 30 Use the Law of Constant Composition to determine if any of the samples could be the same substance. Use the Law…arrow_forward16) What's the atomic number of sulfur-34 ? A) 34 B) 18 C) 16 17) In the formula of hydroxylapatite Cano(PO4)6(OH), determine the number of each kind of atom, Ca, O, H, P: A) Ca – 10; O – 8; H – 2; P - 6 с) Са — 10; О —12;B Н — 2;B Р - 6 в) Cа — 10; О — 26;B Н — 23B Р - 6 D) Ca – 10; O – 26; H – 2; P - 1 18) Which element is represented by the chemical symbol TI ? A) tantalum B) thorium C) thallium 19) The fact that water is 11.2 % hydroen and 88.8 % oxygen by mass regardless of its origin is an example of B) law of conservation of mass C) law of definite proportions A) Dalton's atomic theoryarrow_forward
- Aunt Julie went to the jewelry store to buy a gift for her amiga. As an aunt from Poveda, she knows that the karat scale is based on mass percentages. She was also informed by Uncle Bruno that pure gold is equivalent to 24 karats. If she bought a bracelet made of gold alloy that is 50 mol% silver and 50 mol% gold, what is the karat number of the bracelet?arrow_forward2. What is the mass percent of iron in Fe(NO3)3 • 9H2O?arrow_forward5 & 6 &7 plzarrow_forward
- Q 22 pleasearrow_forwardNaturally occurring silver consists of 107Ag (106.905 u) and 109Ag (108.905 u). What is the relative abundance of 109Ag? Question 11 options: 51.84% 48.16% 50.00%arrow_forwardA mass spectrometer is being used to separate common oxygen-16 from the much rarer oxygen-18, taken from a sample of old glacial ice. (The relative abundance of these oxygen isotopes is related to climatic temperature at the time the ice was deposited.) The ratio of the masses of these two ions is 16 to 18, the mass of oxygen-16 is 2.66 ✕ 10−26 kg, and they are singly charged and travel at 7.40 ✕ 106 m/s in a 1.40 T magnetic field. What is the separation (in m) between their paths when they hit a target after traversing a semicircle?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY