
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Please help!!
1) 42 g of OCl2
2) 4.30 moles of CCl4
3) 3.76 × 10^34 molecules of SeCl4.
Calculate the no. Of chlorine atom in each of the sample.
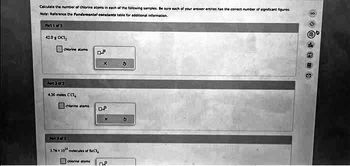
Transcribed Image Text:Calculate the number of chiorine atoms in each of the following samples. Be sure each of your answer entries has the correct number of significant figures
Note: Reference the Fundamental constants table for additional information.
Part 1 of 3
42.0 OCI,
chlorine atom
Put 28/3
430 moles CC1,
chionine ations
De
of
3.76x 10 molecules of BC1,
choreo n
00
die
M
8
S
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- chloral hydrate (C2H2Cl3O2) is a drug formerly used as a sedative and hypnotic. It is the compound used to make "mickey Finns" in detective stories. Calculate the molar mass of chloral hydrate.arrow_forwardC,Hale + 50, 'a(g) 2(e) →3 COe + 4 H,0e 2(g) How many moles of 0, are required to produce 17.8 moles of CO,?arrow_forwardChlorine reacts with phosphorus to form a compound Q with relative molecular mass of 137.5. The compound contains 77.5 % of chlorine by mass. (a) Deduce the empirical formula of the compound Q. (Relative atomic masses: P = 31.0, Cl = 35.5) Constituent atoms P Cl % by mass Relative atomic mass Relative no. of mole of atoms Simplest mole ratio of atoms The empirical formula of compound Q is ____________________ (b) Find the molecular formula of Q. (c) Draw the electronic diagram of the compound Q, showing electrons in the outermost shell only.arrow_forward
- Calculate the mass in g corresponding to 3.400 mol H2Oarrow_forward3.B) Determine the number of moles and mass of Al(OH)3 produced. Al2(SO3)3 + 6NAOH -> 3Na2SO3 + 2Al(OH)3arrow_forwardMany explosives have the general formula CwHxNyOz where w, x, y, and z are subscripts. These explosives decompose according to the equation:CwHxNyOz --> w CO2 + x/2 H2O + y/2 N2Which equation allows the subscript z (the number of oxygen atoms) to be calculated?arrow_forward
- A compound has two elements A and B. If the molar ratio of elements A : B is 1: 1.5 respectively; what formula? would be its empirical O A. AB1.5 О В. АдВ6 O C. AB O D. A2B3arrow_forwardConvert 40.0g K3PO4 to molecules of K3PO4 . choose the correct multiple choice underneath. 40.0 molecules 1.14x10^23 molecules 1.14x10^23 g 1.14x10^23 molesarrow_forward1. In a certain compound of copper and oxygen, CuxOy, we find that a sample weighing 0.6349 g contains 0.5639 g of Cu. What is the mole ratio (number of moles Cu/number of moles O) in the sample? Write the resulting Empirical Formula. 2. In a certain compound of chromium, chlorine, and water, CrxClyoz H₂O, we find that a sample weighing 20.0000 g contains 3.9082 g of Cr and 8.1136 g of water. What is the mole ratio (number of moles Cr/number of moles Cl and number of moles Cr/number of moles of H₂O) in the sample? Write the resulting Empirical Formula. 3. A carbohydrate sample weighing 5.0000 g with a formula of CxHyO₂ is dehydrated to leave 2.0001 g of C. What is the mole ratio (moles of C/ moles of water) in the sample? Write the resulting Empirical Formula.arrow_forward
- MOLES/MOLECULES T04/S03 If a sample of B10H12[N(CH3)3l2 is found to contain 7.48 · 1020 B atoms how many N atoms are present? Express your answer in scientific notation. Molar Mass (g/mol) B10H12[N(CH3)3]2 238.43 C atom B atom N atom 12.011 10.811 14.007 х 10 H atom Avogadro's No. (mol"1) 6.022x1023 1.0080arrow_forwardHW 1-Calculate the mass of compound required to prepare 100.00 mL of a 1.40x102 M CuCl, ?. www.arrow_forwardQuestion #5arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY