
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
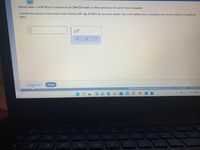
Transcribed Image Text:Normal saline, a 0.90 %(wv) solution of salt (NaCl)in water, is often used as an IV carrier fluid in hospitals.
Calculate the volume of this solution that contains 345. mg of NaCl. Be sure your answer has a unit symbol and is rounded to the correct number of significant
digits.
x10
Explanation
Check
O 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Accessibility
W
T
Pouo
PriScr
nsert
Delete
F10
F11
F12
F6
F7
F8
&
E
R
T
H
K
D
CO
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Question 7 Consider the following prescription: Promethazine HCI 62.5 mg Nacl qs SWFI ad 50 mL Make isoton. soln. The E-value of promethazine HCl is 0.18. How many milligrams of sodium chloride should be used in compounding the prescription? (Your answer must be numeric. Do not include units. Round to the nearest whole number.) 1200arrow_forwardReport 2 decimal places for all your answers. Do NOT include units in your answer. BaCl₂ H₂O 208.23 Mass of anhydrous BaCl₂ (amu) Mass of H₂O molecules in Hydrate (amu) Mass of Hydrate (amu) Percent H₂O in Hydrate (%) O BaCl 208.23 0.00 At n 208.23 plete the table. 0.00 % 18.02 226.25 7.97% Select one: O a. BaCl, • H2O O b. BaClz • 2 H2O O c. BaCl2 • 3 H2O O d. The data is inconclusive: it could be either BaCl₂ H₂O OR BaCl₂.2 H₂O O e. The data is inconclusive: it could be either BaCl₂ 2 H₂O OR BaCl₂ 3 H₂O BaCl₂ 2 H₂O 208.23 51°F 36.04 244.27 14.8 0 % Compare the experimental percent water you calculated for the barium chloride hydrate in the Question 6 in this assignment (above) with the theoretical percent water values calculated from the possible formulas in the table above. Do you think that the formula for hydrate is BaCl₂ H₂O, BaCl₂ 2 H₂O, or BaCl₂3 H₂O? End BaCl₂ 3 H₂O 208.23 PgUp 54.06 262.29 20.6 40) % J 6 Manage 9:44 PM 10/9/2022 PgDnarrow_forwardestion 10 A solution of methanol (CH3OH, MM - 32.042 g/mol) dissolved in ammonia (NH3. MM - 17.034 gimol) has a concentration of 5.1 m. What is the mole traction of the solute Report your answer to three decimal places.arrow_forward
- soution B has more sugar that solution A. Solution B has 30%sugar and solution A has 10% sugar. what are the percentage of water? A. A= 10% water, B= 30% water B. A= 20% water, B= 60% water C. A=80% water, B=40% water D. A=90% water, B=70% waterarrow_forwardAchemist adds 1.35 L of a 1.72 mol/L sodium thiosulfate (Na₂S₂O3) solution to a reaction flask. Calculate the mass in kilograms of sodium thiosulfate the chemist has added to the flask. Be sure your answer has the correct number of significant digits. kg Explanation Check 80 x10 X 888 S FS MacBook Air FO ? DI FR olo 18 Ar 8- 11242 © 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility I F11arrow_forwardMacmillan Learning A solution of CaCl₂ in water forms a mixture that is 28.5% calcium chloride by mass. If the total mass of the mixture is 631.1 g, what masses of CaCl, and water were used? mass of CaCl₂: mass of water: g garrow_forward
- ||| O STOICHIOMETRY Using molarity to find solute mass and solution volume A chemist adds 90.0 mL of a 1.41M aluminum chloride (AIC13) solution to a reaction flask. Calculate the mass in grams of aluminum chloride the chemist has added to the flask. Be sure your answer has the correct number of significant digits. g Explanation Check . === x10 X S JUL 19 4 m 0/3 tv tv♫♬ Kirst 2022 McGraw Hill LLC All Dinhte Docenied Torme of Lleo Privacy Center | Ac Nall A J N.arrow_forwardPart D: number of moles?arrow_forwardA chemist prepares a solution of magnesium fluoride (MgF,) by measuring out 9.9 mg of magnesium fluoride into a 350. mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in mol/L of the chemist's magnesium fluoride solution. Round your answer to 2 significant digits. mol L x10 ? Explanation Check O 2022 McGraw Hill LLC All Rights Reserved. Terms of Use Privacy Center Accessiblty 80 F Cloudy DELL PriScr Delete Insert F11 F12 F5 F6 F7 F8 F9 F10 F1 F2 F3 F4 Hum. Lock 44 Backspace & 8. %24arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY