
Predicting the Water Solubility of a Compound
Which compounds are water soluble?
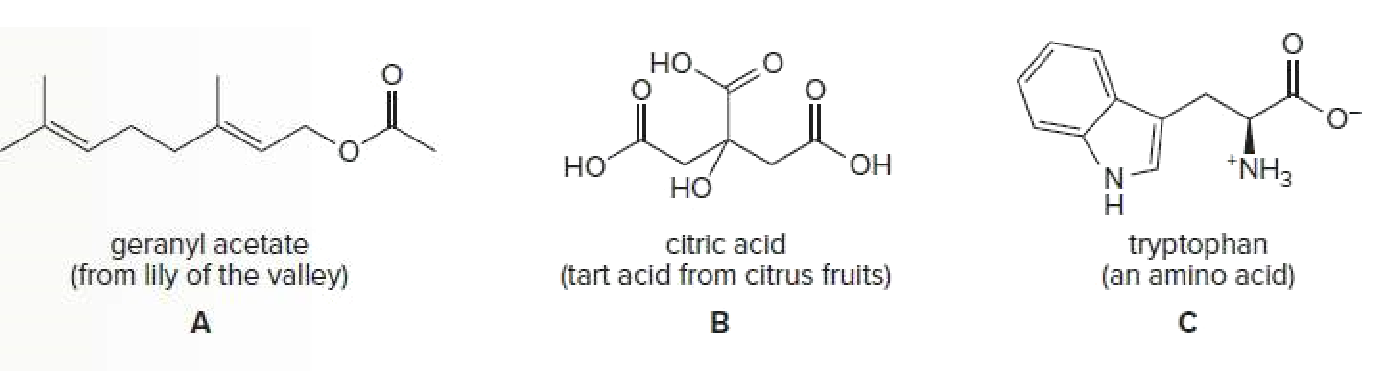
a) Geranyl acetate is a water-insoluble compound because it contains more hydrophobic part
due to bulkier hydrocarbon, its polarity is very less hence insoluble in water but soluble in the organic compound
b) Citric acid is soluble in water because it is polar in nature due to the acidic group and hydroxy group
The more the molecule is polar in nature more it is soluble in water because water is polar in nature.
like dissolve like
c) Tryptophan is very slightly soluble in water due to the presence of acid and amine group make it polar but due to the presence of indole ring make it non-polar aromatic amino acid
Tryptophan is considered insoluble in water
Step by stepSolved in 2 steps

- Give the IUPAC name for the compoundarrow_forwardQuestion 1: a) Identify each of the functional groups below by name. H3C CH3 CH3 H CH3 Alkane MOST REDUCED H3C N Alcohol H3C Aldehyde я HN SCH₂CH3 NH b) The oxidation state of the carbon atom increases as shown in the scheme below. Organize the functional groups from question 1a according to the increasing oxidation state of their most oxidized carbon atom. Group all functional groups with at same oxidation state into groups and match them with their counterparts in the provided scheme. H-N-CH₂ `N+ CH3 Carboxylic acid H MOST OXIDIZED Carbon dioxidearrow_forwardUsing IUPAC rules as described in class, supply the name or formula of the compound, as needed. periodic acid HBr(g) Sr(HSO4)2 zinc permanganate 1207arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





